Company of the week: Elevara Medicines
Company Overview and Corporate Structure
Elevara Medicines Ltd. is a London-based clinical-stage biotechnology company focused on innovative therapies for rheumatoid arthritis (RA) and related inflammatory diseases. The company was co-founded in 2025 by Weatherden, a UK clinical development advisory firm, together with venture investor Monograph Capital.
Weatherden's involvement provided Elevara with seasoned drug development expertise from day one – notably, Elevara's CEO Emma Tinsley concurrently leads Weatherden, reflecting a deliberate incubation model. Elevara's headquarters are in London, UK, and it operates as a privately held entity (Elevara Medicines Limited) under UK jurisdiction.
Leadership and Board
Elevara's leadership team combines biotech industry veterans and rheumatology experts. CEO Emma Tinsley has a background in biotech finance and clinical strategy, having served as CFO and then CEO of Weatherden since 2017.
The Chief Medical Officer (CMO) is Prof. Dominique Baeten, M.D., Ph.D., a prominent rheumatologist and professor at Amsterdam UMC since 2012. Baeten brings deep immunology and clinical trial expertise, having long researched chronic arthritis pathogenesis.
Other key team members include Geoff Brooks (Head of Clinical Operations), Dr. Kirsty Wydenbach (Head of Regulatory Affairs), Dr. Simon Hutchings (Head of Clinical Pharmacology), and Dr. Margaret Jones (Head of Biometrics) – indicating a strong emphasis on clinical trial execution and data science. Notably, many of these leaders have prior regulatory and development experience (for example, Dr. Wydenbach previously served at the MHRA), underscoring Elevara's competency in navigating trials and approvals.
Elevara's Board of Directors is investor-heavy, reflecting its venture-backed status. In addition to CEO Tinsley, the board includes Tim Funnell, Ph.D. (Monograph Capital), Maina Bhaman (Sofinnova Partners), Vanessa Carle, Ph.D. (Forbion), and Gijs van den Brink, M.D., Ph.D. as an independent director. This composition aligns with the Series A financing structure (co-led by Forbion and Sofinnova) and ensures investor oversight. The presence of seasoned VCs and an independent expert on the board provides governance and strategic guidance, which will be crucial as the company advances its pipeline.
In summary, Elevara's corporate structure is that of a young venture-funded biotech with operational roots in a specialist consultancy (Weatherden). This gives it a lean startup profile but with an unusually experienced team for its stage. The headquarters in London situates it within the UK's biotech scene, benefiting from local biomedical talent and networks, while its board and investors provide international reach (e.g. Forbion from the Netherlands, Sofinnova from France). This blend of clinical/regulatory expertise and strong investor backing positions Elevara with a solid foundation to execute its ambitious clinical plans, albeit within the constraints of a private startup needing to prove itself in the coming trials.
Clinical and Preclinical Pipeline Analysis
Lead Program ELV001 – Targeting Fibroblasts in Rheumatoid Arthritis
Elevara's flagship program is ELV001, a first-in-class, oral cyclin-dependent kinase 4/6 (CDK4/6) inhibitor being developed as a novel disease-modifying antirheumatic drug (DMARD) for rheumatoid arthritis.
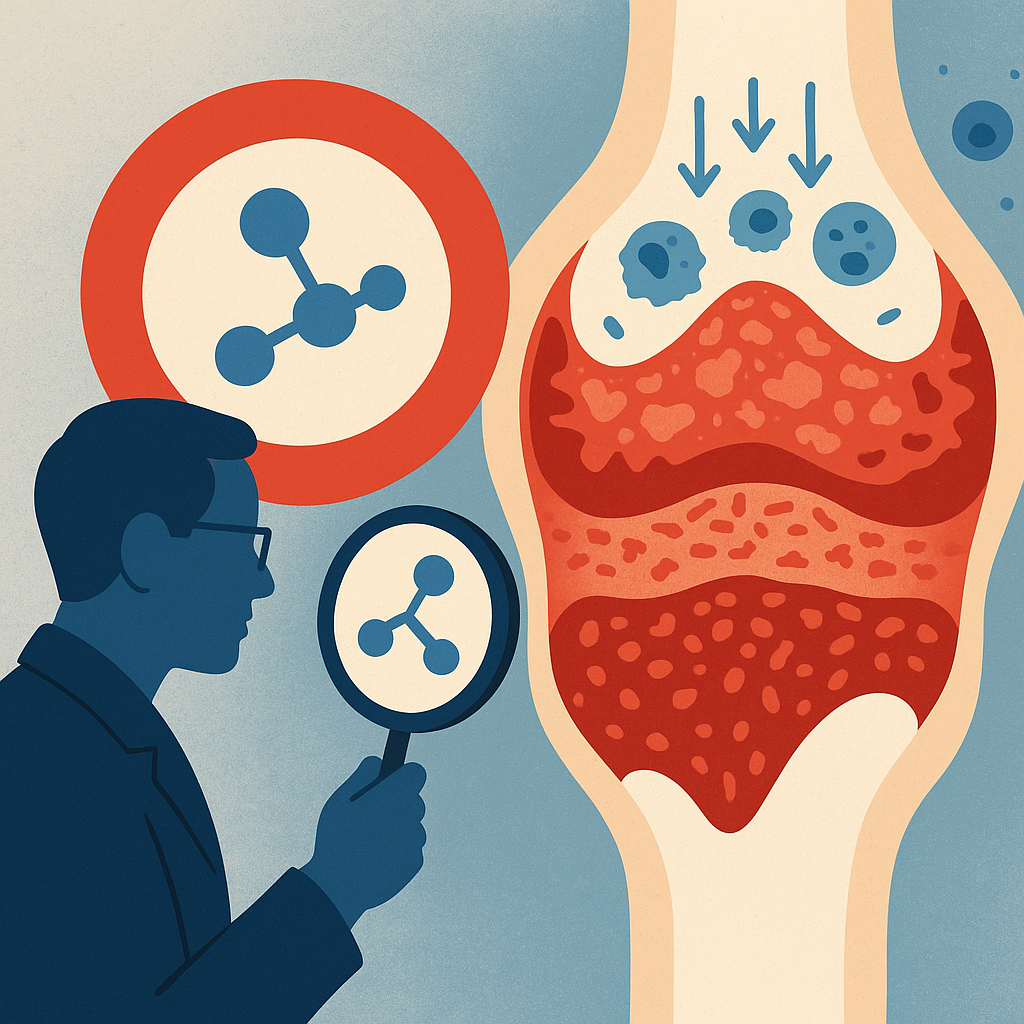
Unlike traditional RA therapies that suppress immune cells, ELV001's mechanism is to selectively target fibroblast-like synoviocytes (FLS) – the resident stromal cells of the joint synovium – which are key drivers of RA pathology. FLS in RA patients exhibit hyperproliferation and aggressive behavior (sometimes likened to "tumor-like" growth in the joint), producing inflammatory mediators and enzymes that degrade cartilage and bone.
By inhibiting CDK4/6, ELV001 aims to halt the abnormal proliferation and inflammatory signaling of synovial fibroblasts without broadly suppressing the immune system. This orthogonal approach addresses RA as not just an immune-driven disease but also a tissue-driven disease, attacking the "rogue" stromal cells that contribute to chronic inflammation.
Mechanism of Action
CDK4/6 are cell cycle kinases; inhibitors of CDK4/6 (such as palbociclib and related drugs) are well-known in oncology for arresting tumor cell proliferation. ELV001 repurposes this concept to target synovial fibroblasts.
Preclinical studies by Teijin Pharma (the originator of ELV001, previously code-named TCK-276) showed that the compound is highly potent and selective for CDK4/6, leading to suppression of FLS hypertrophy and pannus formation in RA models. By inducing cell-cycle arrest in FLS, ELV001 is expected to reduce the production of inflammatory cytokines (like IL-6, IL-8), matrix metalloproteinases, and tissue-degrading enzymes that FLS secrete, thereby mitigating joint inflammation and damage at the source.
Importantly, Elevara emphasizes that ELV001's action is local and non-immunosuppressive – it does not directly inhibit T-cells, B-cells or other immune effectors. This could avoid the systemic immune suppression seen with current RA drugs (e.g. TNF blockers, JAK inhibitors), theoretically reducing infection risk and other side effects. Mechanistically, the selectivity comes partly from pharmacokinetics: ELV001 has a relatively fast elimination half-life (6–12 hours) and did not accumulate significantly with daily dosing. This rapid clearance might spare bone marrow and immune cells from sustained CDK4/6 inhibition, a hypothesis supported by early safety data.
Clinical Development to Date
ELV001 was originally discovered and developed through Phase 1 by Teijin Pharma in Japan. Teijin conducted Phase 1 trials in the United States, including a single-ascending-dose study in healthy volunteers and a multiple-ascending-dose Phase 1b in RA patients.
The Phase 1b trial (NCT05437419) enrolled 32 patients with active RA (on stable background therapy) to receive ELV001 (TCK-276) or placebo once daily for 7 days. The published results demonstrated a favorable safety and tolerability profile for ELV001 up to the highest tested multiple dose (175 mg/day for 1 week).
Notably, no serious adverse events or dose-limiting toxicities were observed. Critically, common CDK4/6 inhibitor toxicities like neutropenia, anemia, or thrombocytopenia were not seen – even transiently – at doses up to 185 mg single dose or 25 mg/day repeated dosing. The absence of such hematologic side effects, which are often observed with oncology CDK4/6 inhibitors, supports Elevara's claim that ELV001 does not cause meaningful systemic immunosuppression.
Teijin's scientists attributed this safety to the compound's pharmacokinetic profile (faster plasma clearance than cancer-focused CDK4/6 drugs) and possibly the lower proliferative index of immune cells relative to synovial FLS.
Even within just 7 days of dosing in the Phase 1b, early signals of efficacy emerged. Treated RA patients showed improvements in standard disease activity metrics compared to placebo. For example, at Day 7, 40–80% of patients on mid-to-high doses of ELV001 achieved a good or moderate EULAR response (DAS28-CRP based), vs. 12.5% on placebo. Similarly, ACR20 response rates (≥20% improvement criteria) were 33–60% in ELV001 groups (dose-dependent) vs. 0% on placebo after just one week.
Although patient numbers were small (6 per dose cohort), these results are encouraging: they indicate a pharmacodynamic effect on RA symptoms even in the short term. Achieving any ACR20 responses in a 7-day trial is unusual, given most RA therapies take several weeks to onset – this hints that ELV001 may have a rapid anti-inflammatory effect on the synovium.
Elevara has also cited preclinical synergy data, noting that combining ELV001 with standard DMARDs (like methotrexate or TNF inhibitors) produced "rapid and robust efficacy" in models. The hypothesis is that by pairing an immune-targeted drug with ELV001's fibroblast-targeted action, a deeper remission can be achieved than either alone – effectively raising the ceiling of efficacy beyond what immune modulation alone provides.
Phase 2 Trial – START-SYNERGY
Armed with the Phase 1b safety/efficacy data, Elevara is now launching a Phase 2 program for ELV001. The upcoming trial is titled START-SYNERGY (Synoviocyte Targeted Anti-Rheumatic Therapy). This will be a global Phase 2b study enrolling approximately 180 RA patients who have inadequate response to standard treatments (methotrexate and TNF inhibitor biologics).
Importantly, these patients are not end-stage refractory cases, but rather the large subset of RA sufferers who have only partial responses to first-line biologics (a very common scenario). Elevara is positioning ELV001 as an add-on therapy for partial responders, rather than a last-resort salvage treatment. In other words, the trial will likely recruit patients currently on methotrexate and a TNF blocker (e.g. etanercept or adalimumab) but who still have active disease, and will randomize them to receive ELV001 or placebo on top of their regimen.
The goal is to demonstrate that ELV001 can "rescue" these patients into remission or low disease activity, validating the fibroblast-targeted approach to "bridge the gap" in the current treatment paradigm.
According to Elevara, START-SYNERGY is expected to begin by December 2025, with trial sites across the United States, South Africa, and multiple European countries (Czech Republic, Poland, Bulgaria, Serbia). Such geographic breadth will ensure a diverse patient population and faster enrollment, while also engaging both FDA and EMA regulatory pathways early on.
The trial design likely includes endpoints such as ACR20/50/70 response rates at 12 or 24 weeks, DAS28 remission rates, and possibly imaging or biomarker endpoints to directly measure synovial inflammation.
Biomarkers
Although specific biomarkers have not been publicly detailed, one can expect MRI or ultrasound assessments of synovitis to see if ELV001 reduces synovial volume or vascularity (given its direct anti-fibroblast effect). Additionally, Elevara might track tissue biomarkers like MMP-3, IL-6, or markers of bone erosion (like CTX-I) in blood or synovial fluid, to see downstream effects.
Another plausible biomarker is cadherin-11, a cell adhesion molecule on FLS that correlates with joint damage; competing fibroblast-targeted therapies (like Roche's anti-cadherin-11) looked at MRI synovitis scores, so Elevara could do similar to show ELV001's impact on joint structure.
Elevara's reference to "synergy" also raises the question of measuring combined efficacy – e.g., showing that ELV001 plus TNF inhibitor yields higher remission rates than historical TNF inhibitor alone. If successful, this would confirm the additive value of hitting FLS.
Beyond efficacy, safety in a longer trial will be closely watched. The Phase 2 will extend ELV001 dosing from 1 week to perhaps 3–6 months, so monitoring for any delayed or cumulative toxicities is critical. While Phase 1b found no hematologic suppression in 7 days, longer exposure could still potentially reveal mild effects on blood cell counts or other off-target issues.
The trial's multi-country nature suggests Elevara has filed or will file multiple regulatory submissions: an IND in the US (likely already in place via Teijin's earlier IND) and Clinical Trial Applications (CTAs) in Europe and South Africa. So far no special regulatory designations (e.g. Fast Track or Breakthrough Therapy) have been announced for ELV001 – RA is a large, competitive field, and such designations usually require significant early efficacy in an unmet subpopulation.
If the Phase 2 shows extraordinary remission rates, Elevara might later pursue Breakthrough Therapy Designation for the subset of RA patients who are partial responders to TNF blockers (a high unmet-need group).
Pipeline Expansion and Exploratory Programs
While ELV001 for RA is the clear focus, Elevara's Series A funding is also earmarked to advance exploratory programs in other chronic inflammatory and women's health indications. The company has not yet disclosed specific preclinical assets or targets, which suggests these programs are in early discovery or concept stage. However, Elevara's strategy and the expertise of its scientific team give clues to likely directions:
Additional Chronic Inflammatory Diseases
Elevara's core concept – fibroblast-driven pathology – could extend to other inflammatory disorders where stromal cells play a role. For example, other forms of inflammatory arthritis such as psoriatic arthritis or ankylosing spondylitis have analogous tissue-resident cells (enthesis fibroblasts, etc.) that sustain inflammation. It's plausible Elevara might test ELV001 or derivatives in such conditions, or develop new compounds that target fibroblasts in those contexts.
Systemic sclerosis (scleroderma) is another fibroblast-mediated disease (excess collagen deposition by fibroblasts causes skin and organ fibrosis); a CDK4/6 inhibitor could theoretically slow fibrogenesis, although systemic sclerosis is a very different pathology from RA. We note that cadherin-11, a fibroblast marker, has been implicated in both RA and fibrotic diseases like idiopathic pulmonary fibrosis and skin fibrosis, hinting at shared fibro-inflammatory pathways. Elevara may explore whether targeting fibroblast proliferation in such diseases yields benefit, though this would be longer-term given the need for distinct clinical programs.
Women's Health Indications
The mention of women's health likely refers to diseases like endometriosis or uterine fibroids, which have significant fibrotic/inflammatory components and high unmet need. In endometriosis, for instance, endometrial stromal cells (fibroblast-like cells) form lesion implants that cause chronic pelvic inflammation and pain.
Research shows activated fibroblasts (e.g., FAP-positive) are present in endometriotic lesions and contribute to lesion fibrosis and immune evasion. If Elevara can adapt its fibroblast-targeting approach here, it could open a novel therapy avenue in a condition that affects millions of women and currently relies on hormone suppression or surgery. An oral non-hormonal treatment for endometriosis that tackles fibrosis would be a major innovation.
Similarly, uterine fibroids (benign tumors of the uterus) involve aberrant fibromuscular cell proliferation – CDK4/6 inhibitors might slow fibroid growth, albeit safety would need careful evaluation in a generally healthy population. Elevara has not confirmed these specific targets, but given the funding mandate, it is reasonable to speculate that preclinical screening or partnership discussions are underway for one or two women's health programs that fit their platform.
Biomarkers and Precision Medicine
As Elevara expands its pipeline, it may also develop biomarker tools to identify patients who would benefit most from fibroblast-targeted therapy. For RA, this could mean finding molecular signatures of "fibroblast-driven" disease – for example, high levels of certain synovial fibroblast markers (cadherin-11, VCAM-1, FAP, etc.) in patient biopsies or blood.
Elevara's CMO Dominique Baeten is an academic expert in immunopathology; under his guidance, the company could integrate biomarker studies into Phase 2 (perhaps performing optional synovial biopsies in a subset of patients to directly observe FLS activity and drug impact). If successful, this could not only validate ELV001's mechanism but also pave the way for a companion diagnostic to stratify patients (enabling a precision medicine approach where those with fibroblast-driven pathology get the drug). Such differentiation would be advantageous in later regulatory and reimbursement discussions.
Overall, Elevara's pipeline beyond ELV001 is in a nascent stage – as a Series A company, it essentially has a single clinical asset and a "to be determined" set of follow-on programs. However, the choice to explicitly mention chronic inflammation and women's health in investor materials signals to investors that Elevara aspires to build a platform around its core insight: that modulating stromal cell biology can treat diseases that traditional immune-centric drugs don't fully resolve.
The company's access to Weatherden's network of clinicians and scientists could facilitate in-licensing additional assets or targets. Indeed, Elevara might pursue more licensing deals analogous to the Teijin partnership – for example, scouting academic research or pharma shelved compounds that affect fibroblast pathways, and then applying their development know-how. This model of "search and develop" served them well with ELV001 and could rapidly expand the pipeline without starting from scratch in the lab.
In conclusion, Elevara's pipeline is anchored by ELV001's mid-stage development in RA, with initial data de-risking the concept, and is likely to broaden into adjacent indications leveraging the same biological rationale. The next 12–18 months will be crucial to see if the exploratory programs solidify (e.g. a second IND in a new indication) or if management wisely focuses resources on making ELV001 a clinical success first. With $70 million raised, they have some bandwidth to push on multiple fronts, but careful prioritization will be needed to avoid diluting their efforts.
Financial Background and Funding Situation
Elevara's financial profile is typical of a high-potential biotech launch backed by prominent venture capital. The company publicly emerged from stealth in October 2025 with the announcement of a $70 million Series A financing. This is a sizable Series A round, reflecting strong investor conviction in Elevara's asset and strategy.
The round was co-led by Forbion and Sofinnova Partners, two well-known life science venture firms in Europe, and included participation from Monograph Capital (the founding seed investor). According to company statements, the financing closed in September 2025 and will fund the Phase 2 trial of ELV001 as well as exploratory research programs. The infusion gives Elevara a multi-year cash runway: likely sufficient to carry ELV001 through Phase 2 readout and to advance at least one additional program into preclinical or early clinical testing.
Investors and Ownership
The Series A syndicate brings a mix of domain expertise and deep pockets. Forbion and Sofinnova Partners are both veteran biotech investors with track records of scaling companies to exit. Forbion (Netherlands-based) specifically has expertise in immunology and previously invested in other inflammation startups, aligning with Elevara's focus. Sofinnova (headquartered in Paris/London) likewise has a history of funding and guiding early-stage European biotechs.
Monograph Capital is a newer venture firm that co-created Elevara – their partner Tim Funnell sits on Elevara's board and presumably was instrumental in sourcing the ELV001 opportunity. The participation of Weatherden (the clinical advisory firm co-founder) in the cap table is a unique aspect; Weatherden likely took an equity stake in exchange for its in-kind contributions to company formation and trial design.
A critical element of Elevara's financing is its strategic partnership with Teijin Pharma. As part of the global licensing deal for ELV001, Teijin received an equity stake of 10% in Elevara and is eligible for milestone payments totaling ~$30 million plus royalties on future sales. This means Teijin is a minority owner and stakeholder in Elevara's success. The 10% equity was likely granted at formation (pre-Series A) as consideration for the asset transfer.
Thus, after the Series A, Elevara's ownership is split among the venture investors (Forbion, Sofinnova, Monograph – likely holding the majority), Teijin (10%), Weatherden/founders, and possibly management/team equity. The exact post-money valuation was not disclosed, but given $70M raised and typical dilution, one could estimate Elevara's post-money valuation in the ballpark of ~$140–$200 million (implied, if investors took roughly 35–50% ownership for $70M). This valuation is robust for a single-asset pre-Phase 2 company, indicating high expectations for ELV001.
Use of Proceeds and Cash Runway
Elevara's cash burn will chiefly go toward the Phase 2 START-SYNERGY trial, which as a global 180-patient study will be a significant expense (potentially on the order of $15–25M, including site costs, drug manufacturing, and overhead). The Series A funds should cover this trial through completion and initial data, expected perhaps by late 2026 or 2027 (assuming 12-month enrollment and 3–6 month treatment duration).
In addition, Elevara will invest in pipeline expansion – early R&D for the chronic inflammation and women's health programs. Those efforts (target validation, preclinical studies) could consume a few million dollars per program per year. Weighing these costs, $70M likely gives Elevara approximately 2–3 years of operating runway (i.e., into 2027) before needing additional funding, assuming no revenue in the interim.
The presence of heavyweight VCs signals that if progress is on track, a Series B or crossover round could be raised when Phase 2 data approaches, potentially at a higher valuation.
Non-Dilutive Funding
There is no indication of government grants or non-dilutive funding in Elevara's history thus far. However, given the disease focus, Elevara might be eligible for programs like the UK Biomedical Catalyst or EU Horizon grants for innovative therapies – something the company could explore to extend its cash runway. Another source could be disease foundation funding (e.g., Arthritis Foundation) if certain translational goals align.
Partnerships and Collaborations
Aside from the Teijin deal (which was essentially an in-licensing), Elevara does not yet have co-development or co-funding partnerships with larger pharma. The company deliberately structured itself to own global rights to ELV001. This is a valuable position: if Phase 2 results are positive, Elevara could either raise further funding to proceed alone or engage in partnership discussions for Phase 3 and commercialization.
Given the RA market is large (~$25–30B annually globally according to industry reports), it's plausible Elevara will partner with a Big Pharma for late-stage development, to leverage an existing commercial infrastructure in rheumatology. Potential partners could include the likes of AbbVie, Pfizer, Roche, or Bristol Myers Squibb – all of whom have established RA franchises but continually seek new mechanisms to extend their portfolio.
The M&A outlook for Elevara is certainly on the table: big pharmaceutical companies have historically acquired mid-stage assets in RA if they show differentiation. For example, AstraZeneca's acquisition of MedImmune (for biologics including an RA antibody) or more recently, Johnson & Johnson's stake in Nimbus's TYK2 program (immune-mediated diseases) illustrate the appetite. If ELV001's Phase 2 data demonstrates a significant efficacy boost for partial responders without added safety burden, Elevara could become an attractive acquisition target in 2027–2028, potentially commanding a premium given it addresses an unmet segment of a blockbuster market.
It's also worth noting Elevara's connection to Teijin may shape its strategic options. Teijin Pharma, while not a global top-10 pharma, is a reputable Japanese pharmaceutical firm. They might have rights of first negotiation or a seat at the table for Japan/Asia commercialization (sometimes licensors retain an option for their home market). Even if Elevara partners or is acquired by another company, Teijin's equity stake and royalty rights ensure they benefit from ELV001's success downstream. This alignment could facilitate further collaboration – for instance, Teijin might assist with manufacturing scale-up (they have experience producing the molecule for Phase 1) or with Asian regulatory trials, which can de-risk and accelerate Elevara's path in those regions.
Financial Risks and Considerations
On the flip side, Elevara's financial situation reflects typical biotech risks. They are currently a cash-burning entity with no revenues, wholly dependent on investor funding. The $70M, while substantial, must be managed prudently across R&D activities. Any delays or expansion of trial scope could inflate costs; for instance, if Phase 2 needs to be extended or if additional studies (such as a Phase 2a/2b split) are required, Elevara might have to spend more than budgeted.
Furthermore, the capital markets for biotech in 2025–2026 are unpredictable – a sector downturn could make raising the next round challenging, especially if interim data are lukewarm. However, the involvement of blue-chip VCs somewhat insulates Elevara (these investors often have reserves for follow-ons if warranted).
Another aspect is valuation management. Elevara will aim to increase its valuation by the time of Series B – likely by achieving clinical milestones (e.g., demonstrating proof of concept in Phase 2) and by fleshing out its pipeline (showing it's more than a one-trick pony). If Phase 2 underperforms, the company could face a "down round" or difficulty securing new capital, whereas a strong Phase 2 could catapult its valuation and even enable an IPO or large partnership rather than a traditional venture round.
Finally, Elevara's lean operating model via Weatherden might allow it to conserve cash. Essentially, some operational functions (clinical trial management, regulatory strategy) may be outsourced or handled by Weatherden's team, reducing the need for Elevara to build those capabilities entirely in-house. This is cost-efficient but also means Elevara is somewhat reliant on an external entity (albeit one run by its own CEO). This unusual arrangement will need clear governance to ensure alignment of incentives (Weatherden as a service provider vs. Elevara's best interests).
In summary, Elevara stands on a solid financial footing for a young biotech, with a war chest appropriate for its mid-term goals and strong venture backing. The partnership with Teijin provided a valuable asset without upfront cash cost (though at the expense of equity and future payouts). The main financial task ahead is to create value by de-risking ELV001 in Phase 2 – success there would likely allow Elevara to either raise substantial new funds or secure a lucrative partnership/exit, while failure would leave the company with diminishing cash and the need to pivot its strategy or pipeline.
Competitive Landscape and Peer Benchmarking
Elevara operates in a highly competitive and well-studied disease area – rheumatoid arthritis – but with a novel mechanistic angle that sets it apart. To understand Elevara's positioning, we should examine the current RA therapy landscape, ongoing developments by peers (especially any targeting similar pathways), and how ELV001's profile compares.
Standard of Care and Efficacy Gaps in RA
RA treatment has advanced dramatically over the past two decades, with multiple biologic and oral therapies available. Standard of care typically begins with conventional synthetic DMARDs (like methotrexate) and, for inadequate responders, escalates to biologic DMARDs (e.g. TNF-α inhibitors such as Humira/adalimumab, Enbrel/etanercept; IL-6 inhibitor tocilizumab; CTLA4-Ig abatacept; anti-CD20 rituximab) or newer targeted oral DMARDs (JAK inhibitors like tofacitinib, baricitinib, upadacitinib). These drugs predominantly target immune system components – for instance, TNF or IL-6 inhibitors blunt inflammatory cytokine signaling, and JAK inhibitors block immune cell activation pathways.
While many patients benefit greatly from these therapies, a substantial fraction do not achieve full disease control. As Elevara cites, only about one in three RA patients on TNF inhibitors achieves sustained remission, and up to 50% continue to have partial responses – meaning active symptoms persist despite treatment.
In clinical practice, if a patient has only a partial response, rheumatologists often "cycle" therapies, switching to a different mechanism (e.g., from a TNF blocker to an IL-6 blocker, or to a JAK inhibitor). However, each switch is essentially trial-and-error, and many patients go through multiple biologics without ever reaching remission. This phenomenon is referred to by Elevara as the "ditching and switching" paradigm, which is unsatisfactory for patients and costly to the healthcare system.
Moreover, all current effective RA drugs work by immunosuppression – which raises infection risk and other immune-related side effects. For example, TNF inhibitors predispose to tuberculosis reactivation; JAK inhibitors carry boxed warnings for serious infections, thrombosis, and malignancy risk due to broad immune inhibition.
Thus, there is a recognized need for therapies that can be added to existing regimens to improve efficacy without compounding immunosuppression. ELV001 is directly addressing this gap: it is conceived as an adjunct therapy to break through the efficacy ceiling while "sparing" the immune system. If it succeeds, it could inaugurate a new class of RA treatments targeting the tissue compartment of disease (the joint synovium) rather than the systemic immune response.
Competing Molecules and Approaches in Development
Elevara's approach – targeting synovial fibroblasts – is unique but not without competition. Other companies and academic groups have also identified the stromal component of RA as an attractive target. Below we discuss some notable competing approaches and how ELV001 differs:
Anti-Cadherin-11 Antibody (RG6125)
A pioneering attempt to hit RA fibroblasts was cadherin-11, a cell adhesion molecule highly expressed on FLS in RA joint lining. Roche/Genentech developed a humanized antibody RG6125 against cadherin-11 and tested it in a Phase 2 trial in RA patients with inadequate response to TNF blockers.
This trial, completed a few years ago, was a key proof-of-concept for fibroblast targeting. However, the results were disappointing – RG6125 was well-tolerated but showed no discernible clinical benefit in RA patients (no improvement in disease activity or synovitis on MRI compared to placebo). Roche subsequently discontinued the program, and RG6125 became an example of the challenges in this approach.
The failure suggested that simply blocking cadherin-11 (which might prevent FLS from forming the invasive lining tissue) was insufficient to alter disease outcomes, or that the disease mechanisms had redundancy. Elevara's ELV001 is different in mechanism – instead of blocking one surface protein, it shuts down cell proliferation and inflammatory function of FLS broadly. This broader hammer might be more effective (akin to how shutting off the engine stops a car rather than just deflating one tire).
Nonetheless, RG6125's fate is a cautionary tale: targeting fibroblasts must translate into tangible clinical improvement – not just a neat scientific idea. Elevara will need to demonstrate that ELV001 can succeed where RG6125 did not, by showing clear additive benefit on top of TNF inhibitors. Encouragingly, ELV001's early data (as noted, ACR20 responses even in a short trial) suggest potential efficacy, but confirmation in a longer trial is needed.
Bruton's Tyrosine Kinase (BTK) Inhibitors
Another category of novel RA treatments has been BTK inhibitors – these target B-cell and myeloid cell signaling. While not directly related to fibroblasts, BTK inhibitors represent a new small-molecule approach in RA that emerged in recent years (with molecules like fenebrutinib by Roche, evobrutinib by Merck KGaA, and branebrutinib by BMS).
The competitive insight here is that dozens of RA trials with new mechanisms have failed despite biological rationale. In fact, at least 11 BTK inhibitors were trialed in RA and none progressed to Phase 3 due to insufficient efficacy (sometimes coupled with safety issues). For example, Roche's fenebrutinib showed activity in RA Phase 2 but not enough to beat existing options, and Merck's evobrutinib pivoted to multiple sclerosis after underwhelming RA results.
This underscores that RA is a high bar: incremental improvements often aren't enough to justify a new drug in such a crowded field. Elevara must show a meaningful efficacy boost (e.g., higher remission rates or faster responses) to be competitive. The BTK saga also highlights that small molecules in RA can face safety issues – some BTK inhibitors had off-target effects. ELV001 will need to maintain its clean safety in larger populations.
On the plus side, if ELV001 can deliver what BTKs could not (i.e., measurable added benefit in partial responders), it will stand out as one of the few successful new oral MOAs in RA since the advent of JAK inhibitors.
JAK Inhibitors (next-generation)
The currently approved JAK inhibitors (tofacitinib, upadacitinib, baricitinib) are effective but carry safety warnings. There are next-gen kinase inhibitors, like TYK2 inhibitors (e.g., deucravacitinib, already approved in psoriasis by BMS), being considered for RA and other autoimmune diseases. TYK2 inhibitors aim to offer a better safety profile (more selective action).
If Elevara's ELV001 is truly devoid of systemic immune suppression, it could have a safety edge even over these next-gen orals. However, until long-term data is in, one must consider that chronic CDK4/6 inhibition could potentially cause subtle immune effects (e.g., on lymphocyte proliferation). Peer benchmarking on safety will involve comparing rates of infections, blood count changes, etc., against those seen with JAK or TYK2 inhibitors.
Any hint of immunosuppression in ELV001 could erode its differentiation claim. Conversely, if ELV001 remains clean, it could be used in combination with these immune-targeted orals, which is a niche no one else occupies yet.
Other Fibroblast-Targeting Biotechs
Elevara is not alone in recognizing fibroblasts as a therapeutic target. A notable peer is Mestag Therapeutics, a UK-based biotech (launched 2021) focusing on "fibroblast-immune interactions" in inflammatory diseases and cancer. Mestag's approach differs: they are utilizing single-cell genomics to identify specific subpopulations of pathogenic fibroblasts and then developing monoclonal antibody therapies to modulate fibroblast signaling.
Mestag has several preclinical programs (e.g., MST-0300, an agonist targeting FAP/LTβR in tumors, and M402, a stromal checkpoint agonist for inflammatory disease). While Mestag's disclosed pipeline in inflammation is earlier-stage than Elevara's, they operate in the same conceptual space.
Big pharma interest in Mestag's platform has been significant: in 2021 Janssen (J&J) signed a collaboration with Mestag for two targets, and in 2024 Merck & Co. (MSD) inked a deal worth up to $1.9 billion in biobucks to collaborate on fibroblast-directed therapies. Under the Merck deal, Mestag will use its RAFT (Reversing Activated Fibroblast Technology) platform to discover new targets, and Merck obtained options to license some resulting therapies.
This validates the field: two pharma giants, J&J and Merck, have essentially "vetted" fibroblast targeting as a promising frontier in inflammation by investing in Mestag. For Elevara, this is both positive and negative. On one hand, it suggests that if ELV001 shows efficacy, there will be a receptive audience among big pharma for partnering or acquisition (since they are already interested in fibroblast solutions).
On the other hand, Elevara will eventually face competition from Mestag's drug candidates if they reach clinical trials for RA or other diseases. Mestag's approach (agonizing or modulating fibroblast signals rather than killing fibroblasts) is different; they might, for instance, try to "re-educate" fibroblasts to be less inflammatory. It's too early to say which strategy is superior.
Elevara has the advantage of being first into Phase 2, potentially first to show clinical proof-of-concept. That lead time is critical – a successful Phase 2 would put Elevara in pole position as the fibroblast-targeted RA therapy, forcing others to catch up or differentiate.
Similar Startups
Other companies to watch include Immune Regulation Ltd. (UK) – working on an immunotherapy (BiP protein) that can induce remission in autoimmune diseases including RA by immune resetting (not fibroblast, but another novel approach). Also, SetPoint Medical (USA) with a bioelectronic device to stimulate the vagus nerve to reduce inflammation in RA.
While these are quite different modalities, they all speak to the search for alternatives to chronic immunosuppression. Elevara's bet is on the fibroblast axis; others are trying neuronal reflexes or immune "reset" pathways. The competitive landscape, therefore, isn't just about fibroblast drugs – it's about any approach that could help refractory RA patients.
From a market standpoint, if Elevara's ELV001 and one of these other novel methods both succeed, they might actually complement rather than directly compete, as combinations or sequencing options.
Big Pharma Pipeline
Major pharmaceutical companies still dominate RA treatment and continue to explore new therapies. For example, BMS and Pfizer have been developing TYK2 inhibitors for lupus/psoriasis and could test them in RA. Roche has a novel IL-17 cytokine blocker in trials (RA hasn't been a big IL-17 target historically due to mixed results, but new angles may be tried). Sanofi and Regeneron are investigating a BTK inhibitor (SAR444727) despite the field's setbacks, and AbbVie has an CD40-CD40L costimulation modulator in development.
None of these directly target fibroblasts, but they represent competition in the broader sense of next-gen RA therapies. Elevara will need to demonstrate that adding ELV001 provides benefit above and beyond what these advanced immunomodulators can do. For instance, if a TYK2 inhibitor plus methotrexate gets partial responders to remission, that could diminish the need for ELV001.
However, given the multifactorial nature of RA, it is likely that some patients will still not respond fully to any single new immune pathway – leaving room for a fibroblast-targeted adjunct.
Scientific Differentiation
The key differentiator for Elevara is that ELV001 inaugurates a new category of RA therapy: a "stromal pathway" drug. If one visualizes RA treatment axes: we have broad immune suppression (corticosteroids), targeted immune cytokine blocking (TNF, IL-6, IL-1, etc.), cell-specific interventions (B cells with rituximab, T cell costimulation with abatacept), and intracellular immune signaling (JAKs).
ELV001 sits in none of these categories – it's targeting the mesenchymal cells in the joint that traditionally were not addressed by those drugs. This scientific strategy is differentiated by design. The concept is supported by growing evidence that RA is not purely an immune-mediated disease, but also an "autonomous joint disease" where the local environment sustains inflammation.
As one review put it, fibroblasts were traditionally considered bit players, but are now known to play a central role in chronic inflammation. Elevara's literature references highlight that by targeting FLS, one can intervene in a part of the disease process that immune-targeted drugs leave untouched – the ongoing joint destruction and swelling driven by these cells.
Another differentiation is ELV001's oral administration. Many advanced RA therapies (biologics) are injectables. An oral pill that is additive could be very attractive to patients and physicians, especially if it can be combined with existing treatments seamlessly. We know from the Phase 1 that ELV001 is a once-daily oral pill. Oral JAK inhibitors already gained traction partly due to convenience; ELV001 could ride that wave but with a distinct MOA that might avoid the safety pitfalls of JAKs.
In terms of efficacy benchmarking, Elevara will aim to show that ELV001 plus a TNF inhibitor can achieve remission rates approaching what currently might only be seen with more aggressive therapy. For context, remission (stringent criteria like DAS28 <2.6) is achieved in perhaps ~30% of patients on TNF inhibitors in trials, and low disease activity in ~50-60%.
If ELV001 can raise remission to, say, 50% and low disease activity to >80% in the combo, that's a big win. Also, speed of onset could differentiate it – the company hints at rapid action (possibly due to directly damping inflammatory cell production in joints). A drug that provides pain and swelling relief within days or weeks (faster than biologics which can take 1-3 months) would be notable.
Lastly, Elevara's differentiation will be reinforced if they show synergy, not just additive effect. This would mean demonstrating that using ELV001 with a TNF inhibitor yields a result greater than the sum of each alone. Early clinical hints of synergy are anecdotal (since patients in Phase 1b presumably were on background meds and saw improvement), but a controlled Phase 2 can quantify this.
Market and Peer Positioning
If we step back, the competitive landscape in RA is crowded with large incumbent players, but innovation often comes from smaller biotechs like Elevara (recall that TNF blockers themselves were biotech innovations in the 1990s). Elevara's potential competitors in the market, if ELV001 reaches approval around, say, 2029, would include:
- Generic biologics (biosimilars) of existing TNFs, IL-6 etc. (which by then will be widely used due to lower cost)
- New branded therapies that may appear in the interim (perhaps an oral TYK2 or other novel immunomodulator if any succeeds in late-stage trials)
- Possibly cell therapies – an emerging idea is using CAR-Tregs (engineered regulatory T cells) for autoimmune diseases, including RA. This is still very experimental, but companies are looking at it. If such one-time cell therapies ever became viable, they could "reset" the immune system. However, that's likely beyond the 5-10 year horizon and would complement traditional treatments rather than replace them initially.
Given those, ELV001's niche would likely be: patients with partial responses to first-line biologics, to be used in combination. No other approved drug is specifically indicated for "add-on to incomplete responders" (though in practice doctors combine DMARDs frequently off-label). Elevara could attempt to get an indication for use in that context, which would set it apart in marketing.
In benchmarking terms, one might compare Elevara to how Rinvoq (upadacitinib) was positioned: Rinvoq, a JAK1 inhibitor, was tested in TNF inadequate responders and showed significant improvement vs placebo, earning approval in that setting. Rinvoq however comes with black-box warnings. If ELV001 can match or exceed Rinvoq's efficacy in TNF failures without the safety baggage, Elevara can argue they offer a better risk-benefit for that segment.
Another peer comparison is sfPRDM1 inhibitors (emerging concept of targeting plasma cells in RA) or GM-CSF inhibitors (e.g., mavrilimumab) – these are also being tried for TNF-inadequate responders. They each have had mixed success (GM-CSF blockade by Sanofi's otilimab failed Phase 3 in RA recently). So far, nothing has definitively cracked the code for those partial responders, leaving Elevara an opportunity.
In conclusion, Elevara's competitive landscape is both challenging and auspicious: RA is littered with failed trials and strong incumbents, but the company's unique FLS-focused approach stands out scientifically. The interest from big pharma in similar science (Mestag deals) validates Elevara's rationale. To maintain an edge, Elevara will want to move quickly (solidify Phase 2 results before competitors catch up) and continue to differentiate via data – particularly emphasizing any advantages in safety and the novelty of its mechanism.
If ELV001 delivers, Elevara could define a new competitive sub-space in RA therapy (fibroblast modulators), effectively creating a new race in which it currently holds pole position.
Regulatory and Development Trajectory
Elevara's regulatory strategy is geared toward efficiently advancing ELV001 through mid-stage trials and laying groundwork for late-stage development in multiple regions. Here we outline the known regulatory steps and future considerations:
IND and Early Regulatory Interactions
Because Teijin Pharma conducted the Phase 1 program for ELV001 in the United States, an Investigational New Drug (IND) application was presumably filed with the U.S. FDA (likely by Teijin or its US subsidiary). The Phase 1 trials were done under U.S. regulatory oversight, meaning there is an existing IND dossier containing preclinical safety data, manufacturing information, and the human study protocols. As Elevara has now licensed the compound, it's likely that this IND has been transferred or replicated for Elevara's sponsorship. Elevara's team, including the Head of Regulatory Dr. Kirsty Wydenbach (with her background from the MHRA), is well suited to handle such transitions.
Going into Phase 2, Elevara will be engaging with regulators in multiple jurisdictions: the FDA for U.S. sites and potentially seeking IND amendments if they significantly change the trial design, and European regulators for trial approval via the Clinical Trials Information System (CTIS) since sites in EU countries (Poland, Czech Republic, etc.) are planned. Additionally, South African Health Products Authority (SAHPRA) will oversee their sites in South Africa. This global approach means Elevara must coordinate regulatory filings and approvals to start the trial roughly simultaneously by end of 2025.
Achieving regulatory clearance in these regions is a non-trivial project, but having Weatherden's infrastructure might help (Weatherden likely has experience running multi-country trials).
Trial Design Alignment
An important regulatory step is aligning on Phase 2 design endpoints with agencies. Elevara is conducting a Phase 2b that could potentially serve as one of the pivotal trials if results are strong (though usually two Phase 3 trials are needed for RA). They might seek FDA feedback on using certain endpoints or subpopulations. For instance, FDA might want ACR20/50/70 and HAQ-DI (disability index) as endpoints, whereas EMA might emphasize DAS28 remission rates.
Elevara's trial name "START-SYNERGY" hints that they are emphasizing combination use. It will be interesting to see if they include a design element to prove synergy (e.g., an arm with ELV001 + MTX vs MTX alone in MTX partial responders, or purely add-on to TNF vs TNF alone). Any such complex design would need upfront regulatory discussion. If not already done, Elevara may request a Scientific Advice meeting with EMA or an End-of-Phase 1 meeting with FDA to ensure the Phase 2 will generate data acceptable for further development decisions.
Special Designations
As of now, ELV001 does not have Breakthrough Therapy Designation (BTD) or Fast Track status – it's early for those, and RA is not typically considered for Orphan designation due to its prevalence. However, if Phase 2 yields very compelling results (for example, substantially higher remission in refractory patients), Elevara could potentially apply for Fast Track to expedite development given the unmet need in that subset.
Fast Track would allow more frequent communications and possible rolling submission of an NDA later. Breakthrough Therapy Designation requires preliminary clinical evidence of substantial improvement over existing therapies on a significant endpoint; it's not out of the question if ELV001 showed, say, a remission rate that's double what historical data show for similar patients. For comparison, FDA has granted BTD in RA before (e.g., baricitinib had BTD for RA at one point when it was being expedited).
Elevara could also pursue Priority Medicines (PRIME) status with EMA if they demonstrate clear advantage for a defined population (e.g., RA patients who failed multiple biologics).
Regulatory Challenges
One challenge Elevara might face is how to integrate ELV001 into the treatment paradigm from a labeling perspective. If Phase 3 trials eventually show efficacy, regulators will decide on an indication. It might be something like "for use in combination with methotrexate in adult patients with moderate-to-severe RA who have had an inadequate response to one or more TNF inhibitors" – a specific niche.
Elevara will want as broad a label as possible, but regulators typically follow the trial inclusion criteria for labeling. So Elevara's choice of target population in Phase 2 and Phase 3 is strategic: target too broadly (all RA patients) and risk a failed trial if effect is diluted; target too narrowly (only those failing TNF) and the approved indication will be narrow. They seem to be focusing on inadequate responders to MTX + TNF, which is reasonable and consistent with many RA drug trials (many JAK inhibitor trials enrolled TNF inadequate responders for approval in that line).
Eventually, if successful, doctors might use ELV001 earlier (say after MTX failure before trying a biologic), but initial approval would likely be in later-line use.
Long-term Development Plan
Should Phase 2 be positive, Elevara would need to embark on Phase 3 trials. Typically, at least two large Phase 3 studies (each ~300-600 patients over 6-12 months) are required for RA approvals by FDA/EMA. That is a huge step up in cost and scale. Elevara would likely seek a partnership or major financing to execute this.
We can anticipate that Phase 3 design might test ELV001 in combination with different background therapies: e.g., one trial on TNF inadequate responders (similar to Phase 2 design), another possibly on JAK inhibitor inadequate responders or MTX inadequate responders. If Elevara can show benefit in one class of failures, expanding to others would make sense.
Another regulatory consideration: given ELV001's novel mechanism, regulators might request specific safety monitoring – for example, because CDK4/6 inhibitors in oncology can cause cardiac effects (QT prolongation) or metabolic effects, Phase 3 might need thorough QT studies or dedicated safety substudies. Elevara's Phase 1 didn't flag issues, but larger longer trials might.
Manufacturing and CMC
On the regulatory CMC (Chemistry, Manufacturing, Controls) front, Elevara will rely on manufacturing processes likely established by Teijin. They hold rights to manufacture as per the license. It's possible Teijin continues to supply the active drug for trials under contract. Teijin's decade of work likely means the compound's synthetic route and formulation are well-developed (a plus for Elevara, less early CMC risk).
But for Phase 3 and commercial, Elevara will need a reliable production. If partnering with a big pharma, that partner would handle scale-up. If not, Elevara might have to invest in tech transfer to a large contract manufacturer. Regulatory agencies will inspect and ensure GMP compliance of production – Elevara must plan accordingly.
Global Market Approvals
Looking further ahead, Elevara (or its partner) would seek approvals in major markets: FDA (USA), EMA (EU), MHRA (UK) (post-Brexit, UK has its own process which might follow EMA decisions), PMDA (Japan), etc. Teijin's involvement suggests a path in Japan. Possibly Teijin retained some role for Japan; if not, Elevara might license it back to Teijin or another Japanese firm for local development. In any case, demonstrating efficacy in diverse populations via the global Phase 2/3 will help in global filings.
Regulatory Risk Factors
There are some risks: If any safety signal emerges (say some hint of malignancy risk since CDK4/6 are cell cycle regulators), regulators could impose holds or demand extensive safety data. For example, long-term inhibition of cell cycle might theoretically affect bone marrow stem cells or even increase risk of hematologic malignancies (though none seen so far). Elevara will need to vigilantly monitor safety and engage transparently with regulators.
Another risk is regulatory perception – RA has many therapies, so the bar for approval is that a new drug must show a clinically meaningful improvement. If ELV001's trial results are statistically significant but modest in magnitude, regulators could be lukewarm. It will be important that Elevara pre-specify clinically relevant endpoints (like proportion of patients achieving remission, which is a tangible goal) to make the case that their drug offers something new.
Post-Approval and Life-Cycle
If approved, regulatory focus will shift to post-marketing surveillance. Being a first-in-class agent, health authorities might require a Phase 4 safety study or registry to track any uncommon adverse events when used widely. Also, Elevara could then pursue label expansions – for instance, if initial approval is in TNF-failure RA, they might do studies in earlier RA (MTX failures) to broaden the label, or even test ELV001 in related diseases (some approvals can be faster if mechanism is similar – e.g., an Supplemental Biologics License Application equivalent for a new indication could be easier if RA data is strong).
In summary, Elevara's regulatory trajectory is in early innings. So far, the company has navigated the transition from Teijin's Phase 1 to initiating a Phase 2 with multinational regulatory engagement. The next big regulatory milestone will be obtaining all the necessary Phase 2 trial approvals by late 2025 (a near-term execution challenge). Beyond that, success in Phase 2 would set the stage for pivotal trial discussions with FDA/EMA – perhaps even exploring expedited pathways if data are impressive.
Regulatory strategy will need to emphasize the unmet need in partial responders to position ELV001 not just as another RA drug, but as a solution in an area where current therapies fail. If Elevara manages this narrative and backs it with solid data, regulators are likely to be supportive of bringing a genuinely novel MOA to patients who need it.
SWOT Analysis – Red Team (Risks) vs. Blue Team (Opportunities)
In this section, we present a balanced SWOT analysis from two angles: a Red Team perspective, highlighting the potential weaknesses, risks, and threats facing Elevara, and a Blue Team perspective, emphasizing strengths, opportunities, and the bull case for the company. This dual analysis will provide a comprehensive view of Elevara's prospects.
Red Team Perspective: Risks and Challenges
Scientific and Efficacy Risks
Elevara's entire hypothesis rests on the premise that inhibiting synovial fibroblasts will translate into meaningful clinical improvements in RA. While early data are promising, this mechanism is still unproven in a Phase 2/3 context. The "fibroblast hypothesis" could fail to deliver efficacy beyond what existing drugs offer – similar to how Roche's anti-cadherin-11 failed to show any added benefit in RA.
RA pathogenesis is multifactorial; it's possible that even if ELV001 suppresses FLS activity, the immune system might still drive inflammation unabated, resulting in marginal effects. Additionally, the Phase 1b was very short-term – we don't know if the efficacy signals will sustain or improve over a longer treatment period. There is a risk that the initial response was transient or a placebo-related artifact.
The Red Team also flags that Phase 2 trials in RA are notoriously risky – many agents show activity in small trials only to flounder in larger ones (the history of RA drug development is littered with Phase 2 failures). ELV001 could join the list if, for instance, its effect size is too small or the variability in patient responses is high. The heterogeneity of RA patients might dilute outcomes; if only a subset benefit and Elevara can't identify them prospectively, the trial's overall results could be negative.
Safety and Clinical Risks
Although ELV001 has so far shown a benign safety profile, new risks may emerge with longer exposure and in larger populations. CDK4/6 inhibitors in oncology cause cytopenias (low blood counts) in a high percentage of patients; Elevara is betting that lower dosing and short half-life avoid this, but chronic daily use for months or years could still lead to cumulative bone marrow suppression.
Even a mild neutropenia or drop in lymphocytes could be problematic when adding to patients already on immunosuppressants – increasing infection risk. Regulatory agencies will scrutinize infection rates; if any safety red flag appears (e.g., opportunistic infections, malignancies, cardiac events), it could derail development.
Also, off-target effects are possible. CDK4/6 are expressed in other tissues; prolonged inhibition might theoretically affect tissue repair or regeneration. For example, could inhibiting fibroblasts impede wound healing? RA patients often have comorbidities, and if ELV001 slows healing or causes other subtle problems, it may limit use.
Another risk: tolerability and adherence. Even if serious adverse events are rare, if ELV001 causes daily side effects (say headaches, fatigue – things one might see with cell-cycle inhibitors), patients might be less willing to take an extra drug on top of their regimen.
There's also the risk of clinical trial failure due to execution or design. The Phase 2 START-SYNERGY trial is ambitious – multiple countries and add-on design. If enrollment criteria or background therapies aren't tightly controlled, noise could obscure efficacy. A placebo effect in RA trials can be substantial, especially on subjective measures like pain; demonstrating a significant delta will require careful patient selection and endpoint choice.
Competitor trials running in parallel could also impact Elevara – for instance, if a new JAK inhibitor study shows exceptional results in TNF failures during the same timeframe, Elevara's trial might look less impressive by comparison (even if run independently, perceptions matter).
Financing and Cash Risks
With $70M raised, Elevara has a decent runway, but biotech funding environments can shift quickly. If macroeconomic conditions or investor sentiment for biotech worsen, Elevara could face challenges raising the next round, especially if interim results are not clearly positive. The company's cash burn will accelerate as it goes into Phase 2 and exploratory programs.
Unforeseen expenses – such as needing to expand the trial size to reach significance, or manufacturing delays requiring investment – could strain the budget. If ELV001's Phase 2 needs to be prolonged or repeated due to a failed primary endpoint, Elevara might find itself in a cash crunch. Additionally, Elevara has future financial obligations: milestone payments to Teijin (up to $30M). While presumably staged at late development or approval milestones, these will cut into the proceeds of any partnership or raise.
The Red Team worries about a financing gap: if Phase 2 data won't read out until 2027, Elevara might need to secure more capital in 2026 to keep operations going. That raise could come at a downround or with onerous terms if data are only mediocre or if markets are cold. Early-stage biotechs often depend on continual positive news to sustain valuation – a single trial setback could leave Elevara undercapitalized.
Dependence on Single Asset and IP Defensibility
Elevara is essentially a one-product company at this stage. This concentration risk means the fortunes of ELV001 determine the company's fate. If ELV001 fails or is delayed significantly, Elevara currently has no other clinical asset to fall back on. The exploratory pipeline is too early to pick up the slack in the near term. This puts enormous pressure on ELV001's success.
Furthermore, intellectual property (IP) defensibility is a consideration. ELV001 (TCK-276) is likely protected by composition of matter patents filed by Teijin, but we don't have details on expiry. Since Teijin worked on it for a decade, the patent could have e.g. a priority date in the mid-2010s, meaning it might expire in the mid-2030s (not far beyond the expected launch if things go perfectly). That compresses the time window for market exclusivity unless patent term extensions or new patents (formulations, combinations) are obtained.
Additionally, competing approaches might circumvent Elevara's IP – for instance, another company could develop a different CDK4/6 inhibitor or a selective CDK4 or CDK6 inhibitor optimized for FLS. While Elevara's exclusive license with Teijin gives it rights to TCK-276, it doesn't prevent other firms from trying similar mechanisms if they can find novel compounds. Given that CDK inhibitors are a known class, the barrier to entry may be lower than for a truly unique target.
A competitor with deep pockets could potentially design a molecule in the same class and get it to trials (though they would have to overcome Elevara's head start and any method-of-use patents Elevara might secure for RA). If Elevara's IP does not robustly cover the concept of fibroblast targeting in RA, a fast-follower risk exists.
Regulatory and Market Hurdles
On the regulatory side, RA is a competitive field, and payers and regulators may demand clear evidence of cost-effective benefit. If ELV001 only provides incremental improvement, insurers might be reluctant to reimburse an added drug on top of costly biologics. The Red Team can envision a scenario where even if approved, market uptake could be slow because physicians might be hesitant to use triple therapy (MTX + TNF + ELV001) unless convinced of major gains.
Elevara will have to navigate not just regulatory approval but also health technology assessments, which in Europe could question the need for this drug if biosimilars (cheap TNFs) and JAK inhibitors are available generically by then. Any misstep in Phase 3 or lukewarm data might lead to a label that limits uptake (e.g., only after failing 2 biologics – a small niche).
Talent and Operational Risks
As a small company, Elevara is reliant on a core team of experts. The dual-role nature of some leaders (e.g., Emma Tinsley splitting duties between Elevara and Weatherden, Dominique Baeten balancing academic role) could stretch management thin. There's a risk of talent attrition or distraction – key people might leave if, for instance, larger opportunities arise or if there are disagreements on strategy. Losing the CMO or CEO at this juncture would be destabilizing.
Moreover, scaling up from a project-mode startup to running global Phase 3 trials requires organizational growth. Elevara will need to hire or contract a lot more personnel (clinical monitors, data managers, etc.). Rapid scaling can be challenging – coordination issues or management bandwidth issues could affect trial quality.
The reliance on Weatherden as an incubator is double-edged: while it offers expertise, Elevara must eventually stand on its own as a company. If Weatherden's priorities shift or if any conflict of interest arises, operations could be impacted. Additionally, being UK-based, Elevara could face hiring competition for experienced biotech staff (the talent pool in London is growing but still limited compared to, say, Boston or San Francisco). Attracting top talent (or even maintaining current team motivation) might require successes that are not guaranteed.
Geopolitical and External Risks
External factors could also pose threats. For instance, geopolitical instability or supply chain disruptions could affect Elevara's trial timeline. Some trial sites are in Eastern Europe – any regional conflict or political instability (e.g., considerations stemming from the Ukraine conflict vicinity) might hinder operations or patient recruitment. Brexit-related regulatory divergence is another minor headache – the UK's MHRA now has separate processes; Elevara might choose not to run UK sites initially due to complexity, which ironically could limit UK data (less of an issue now, but for eventual UK marketing authorization it might be relevant).
Another geopolitical angle: If any investor (current or future) is international, say from regions with capital restrictions, funding flows could be impacted by global politics. While Elevara's current investors are Western VCs, future funding could come from sovereign funds or the like, introducing potential complications.
Lastly, the Red Team notes market sentiment risk: Elevara's narrative rides on being innovative. If the broader biotech world sees a couple of high-profile failures in similar approaches (for example, if Mestag's partnerships don't pan out or other fibroblast-target programs stumble), it might sour sentiment towards the whole idea of fibroblast targeting. That could indirectly hurt Elevara's perceived value, making investors skittish or partners less eager.
In sum, from the Red Team viewpoint, Elevara must overcome: unproven mechanism risks, the need to show strong data where many have failed, potential safety unknowns with chronic CDK inhibition, the all-eggs-in-one-basket vulnerability, fundraising challenges if the road gets bumpy, and execution demands that stretch a small organization. The next few years will test whether Elevara can de-risk these red flags one by one – by delivering robust Phase 2 results, securing enough cash, and maintaining flawless operational execution. Any significant stumble on these fronts could significantly set back or even imperil the company's mission.
Blue Team Perspective: Strengths and Upside Opportunities
Strong Scientific Rationale and Novel Mechanism
Elevara's core strength is the compelling scientific rationale underpinning ELV001. The company is built on a growing body of evidence that targeting synovial fibroblasts can address a fundamental gap in RA treatment. Unlike me-too drugs or marginal improvements, ELV001 represents a paradigm shift: treating RA as partly a tissue-driven disease. This positions Elevara as a potential first mover in a new class of therapies.
The Blue Team emphasizes that the Phase 1b data already lend human proof-of-concept – seeing any clinical responses in RA patients within one week is extraordinary. This early efficacy, combined with a clean safety profile (no immune suppression signals), gives ELV001 a validated platform to build on.
In biotech, having de-risked Phase 1 data for both safety and some efficacy is a huge asset going into Phase 2. It means Elevara can pursue aggressive development with confidence that the mechanism is active in humans. The science is further bolstered by Teijin's decade of rigorous work (the "exceptional data package" Elevara's CMO referenced). In essence, Elevara has inherited a jewel that was polished in a pharma setting – a potent, selective molecule with extensive preclinical validation and optimized properties. This is a key strength over many startups that may be working with unvalidated or less mature compounds.
First-in-Class Advantage and IP Position
As the first mover in fibroblast-targeted RA therapy, Elevara stands to gain a significant competitive edge if they succeed. They have locked up the global rights to ELV001, and through that, likely have access to Teijin's patents and can file new patents (for combination use, specific dosing regimens, etc.). The IP, while it has a finite life, is still solid enough to give Elevara exclusivity into the 2030s, and patent term extension (in the US) could add ~5 years beyond approval.
Being first-in-class also often confers a market prestige and pricing power – payers and prescribers tend to value a genuinely new mechanism that can help patients who had no options. If Elevara can demonstrate unique benefits (e.g., remission in X% of refractory patients), they will be in a strong position to justify premium pricing and market adoption.
Moreover, first-mover status means Elevara can set the standard on how to target fibroblasts. They will gather the largest dataset first, which becomes a reference point that competitors must match or beat. With an expanding patent estate (they can patent discoveries like biomarkers predicting response, methods of patient selection, etc.), Elevara can build a moat around the concept of fibroblast-directed treatment in RA and potentially other diseases.
Experienced Leadership and Operational Excellence
Elevara's team is a notable strength. CEO Emma Tinsley and the Weatherden connection mean that clinical development expertise is baked into the company's DNA. The Blue Team sees the Weatherden co-founding not as a conflict, but as a clever way to bootstrap high-quality operations. Elevara likely has access to a pre-existing infrastructure for trial design, regulatory affairs, and project management via Weatherden. This reduces the typical execution risk that plagues young biotechs.
CMO Dominique Baeten brings world-class rheumatology insight – he knows what endpoints matter and what pitfalls to avoid in trial design, and he can interface with key opinion leaders (KOLs) to champion the approach. The rest of the leadership, with heads of regulatory, clinical pharmacology, etc., indicates Elevara has in-house the critical skills to navigate trials and filings, rather than learning on the fly. This gives confidence that Elevara will execute Phase 2 smoothly and generate high-quality data.
The Board composition (with Sofinnova and Forbion principals) is also a plus; these investors have shepherded many companies through growth and will ensure Elevara remains focused and funded. Essentially, Elevara punches above its weight class in terms of know-how, thanks to assembling a team one might expect at a larger biotech. This could result in faster progress and fewer mistakes, accelerating time to key milestones.
Financial Backing and Investor Quality
With $70M in the bank from top-tier VCs, Elevara is well-capitalized for a private Series A company. The caliber of investors (Forbion, Sofinnova) provides not just money but networks and strategic guidance. These investors can help Elevara with partnering discussions when the time comes, and even interim financing if needed.
The Blue Team notes that raising such a large Series A in 2025 signals that investors see potential for a high-value exit, potentially in the hundreds of millions or more if Phase 2 is successful. This kind of war chest also means Elevara can expand its pipeline or double down on success without waiting for external funding. For example, if interim Phase 2 data at some point look good, Elevara could immediately initiate a second Phase 2 in another indication (like psoriatic arthritis or endometriosis), accelerating value creation on multiple fronts.
Additionally, Teijin's equity stake aligns a big company's interest with Elevara – Teijin, with its corporate resources, wants to see Elevara succeed and could be a bridge to the Asian market or even a future acquirer. All these financial aspects suggest Elevara is in a robust position to seize opportunities and weather challenges better than a typical one-asset startup.
Positive Clinical Momentum and Near-Term Milestones
Elevara is entering a phase with rapid, value-inflecting milestones. The Phase 2 trial is set to start imminently (end of 2025), meaning that by perhaps late 2026 or early 2027, initial results could read out. For the Blue Team, this is a short horizon to a significant proof point. If that trial shows even moderate success – say a clear efficacy signal and safety confirmation – Elevara's valuation and strategic options will multiply.
They could likely raise a Series B/crossover at a much higher valuation or even consider an IPO. In the bull case, Elevara could follow the path of other successful mid-stage biotechs: e.g., go public and raise $100M+ on Phase 2 data, or strike a partnership with a big pharma for Phase 3 with an upfront payment.
The proximity of such events is an opportunity; Elevara doesn't face a decade of preclinical work – they are in the clinic and advancing. This attracts interest from investors and partners early. Notably, big pharma scouts are surely aware of Elevara given the uniqueness – Elevara could be on a trajectory where multiple pharma start courting them after Phase 2 data, creating a competitive bidding scenario. That could lead to a very favorable partnership or acquisition deal (the Blue Team envisions perhaps a $500M+ upfront type deal if Phase 2 data are stellar and multiple bidders emerge, given the RA market size and novelty).
Pipeline Expansion and Versatility
While the Red Team saw one-asset risk, the Blue Team argues Elevara's platform has broad potential beyond RA and the company is already positioning to exploit that. The women's health angle, for example, opens doors to indications like endometriosis, which affects ~10% of women and lacks effective non-hormonal treatments. If Elevara can apply ELV001 or a similar approach there, it could tap into a large, underserved market.
Also, chronic inflammatory conditions – we can envision Elevara exploring fibrosis-related diseases (e.g., pulmonary fibrosis or even fibrotic manifestations in systemic lupus or others) using their knowledge of fibroblast biology. The flexibility of a small molecule like ELV001 means it could potentially be tested in any condition where pathological fibroblasts play a role. The company could rapidly generate proof-of-concepts by doing smaller Phase 2 trials in these niches. Each success would represent a new asset in Elevara's portfolio, multiplying company value.
This diversification is already hinted by their funding allocation and statements. Moreover, Elevara's approach may yield synergies with other therapies – for instance, combining fibroblast inhibition with immunotherapy might be useful not only in RA but even in cancer (where cancer-associated fibroblasts hinder immune cell infiltration). While Elevara hasn't stated oncology intentions, the science is not far-fetched. If down the line resources allow, they could partner with an oncology-focused group to test ELV001 or derivatives in tumors known for fibrotic stroma (like desmoplastic pancreatic cancer). Such a move would tap into entirely new investor and partner pools, possibly increasing upside dramatically.
Undervalued Assets and Hidden Value
The Blue Team believes Elevara's current valuation (implicitly in the low-mid hundreds of millions) may undervalue some hidden assets. For one, the Teijin partnership not only gave Elevara ELV001 but also a trove of data and perhaps additional chemical matter (Teijin might have had backup CDK4/6 compounds or related research). Elevara could have rights to these or at least the inside knowledge, which is a form of intellectual asset.
Additionally, Elevara's team (especially Baeten's academic ties) could bring in biomarker programs or investigator-initiated studies at low cost, generating extra knowledge that others don't have. If Elevara identifies a biomarker that predicts response to ELV001 (say a gene expression signature of aggressive FLS), that biomarker itself is valuable IP – it could be used for patient selection not only for ELV001 but for any fibroblast-targeting therapy (meaning Elevara could license that test even to others).
Another undervalued aspect is market positioning: if ELV001 works, Elevara would have a foot in both the rheumatology market and possibly in adjacent autoimmune markets. The RA market, as noted, is expected to approach $40–60B by 2030. Capturing even a small slice (a new drug that takes 5% share of that market is a blockbuster).
But beyond RA, conditions like psoriatic arthritis, systemic sclerosis, or even osteoarthritis (a bit speculative) could expand the addressable market. The company's name "Elevara Medicines" (plural) suggests they intend to have multiple shots on goal. A single success with ELV001 would provide cash (through partnership or revenue) that could fuel those expansions, essentially compounding Elevara's growth. The current Series A valuation likely prices only the RA opportunity; thus any hint that the pipeline is expanding (e.g., announcing a second program entering clinic) could be pure upside not currently reflected.
Regulatory and Commercial Tailwinds
On the regulatory front, if ELV001's data are compelling, Elevara could enjoy some regulatory tailwinds. For example, they might obtain Breakthrough Therapy Designation which would speed up development and review, potentially putting them on market faster than expected. Faster approval saves money and extends patent life on market.
Commercially, the timing could be favorable: by the time ELV001 is ready to launch (~2029-2030), many original biologics (like Humira, Enbrel) will be generic/biosimilar. While that means cheaper options are around, it also means the market will be looking for differentiation – new brand drugs will need to offer something novel beyond what those older drugs (now commodities) do. ELV001 fits that need perfectly – it's not "just another IL-6 or JAK," it's something truly new.
Payers might appreciate that it helps patients who otherwise would cycle through several costly biologics, potentially reducing downstream healthcare costs (fewer joint replacements or hospitalizations due to better disease control). Elevara can make a health economics case that adding ELV001 early for partial responders prevents long-term joint damage and disability, which has economic value. This could ease reimbursement discussions and support wide adoption.
Strategic Flexibility
Elevara also has strategic flexibility due to its strong funding and relationships. If needed, they can pivot or partner cleverly. For instance, if Phase 2 shows ELV001 works best in combination with a certain drug, Elevara could partner with the maker of that drug to co-market or co-develop for that sub-population. Their investors have broad portfolios; Sofinnova or Forbion might have other portfolio companies or connections where synergies exist (for example, Sofinnova has invested in many immunology companies – there could be crossover trials possible).
In a bull scenario, Elevara could even spin off a separate indication into a new company (leveraging the Monograph Capital model to seed another venture with fibroblast tech in, say, fibrosis), creating additional value streams.
Member discussion