Longevity Treatments in Human Trials (2024–2025)

The field of longevity therapeutics has rapidly progressed from animal studies to human clinical trials in recent years. Multiple approaches – from senolytics to gene therapy – are being tested in clinics around the world. Figure 1 illustrates the geographic span of these trials (primarily in the US, with some in Europe, Asia, and South America), and Figure 2 shows the variety of therapeutic targets under investigation. The sections below detail each therapy, linking to sources (including clinical trial identifiers where available), current trial phases, expected readouts, participant numbers, and key findings to date.
Figure 1: Global locations of major longevity trials in 2024–2025. Red dots indicate trial sites (USA has multiple clusters; EU planned sites; trial in Singapore; a paid gene therapy in Colombia) based on sourcesafar.orgclinicaltrialsarena.com.
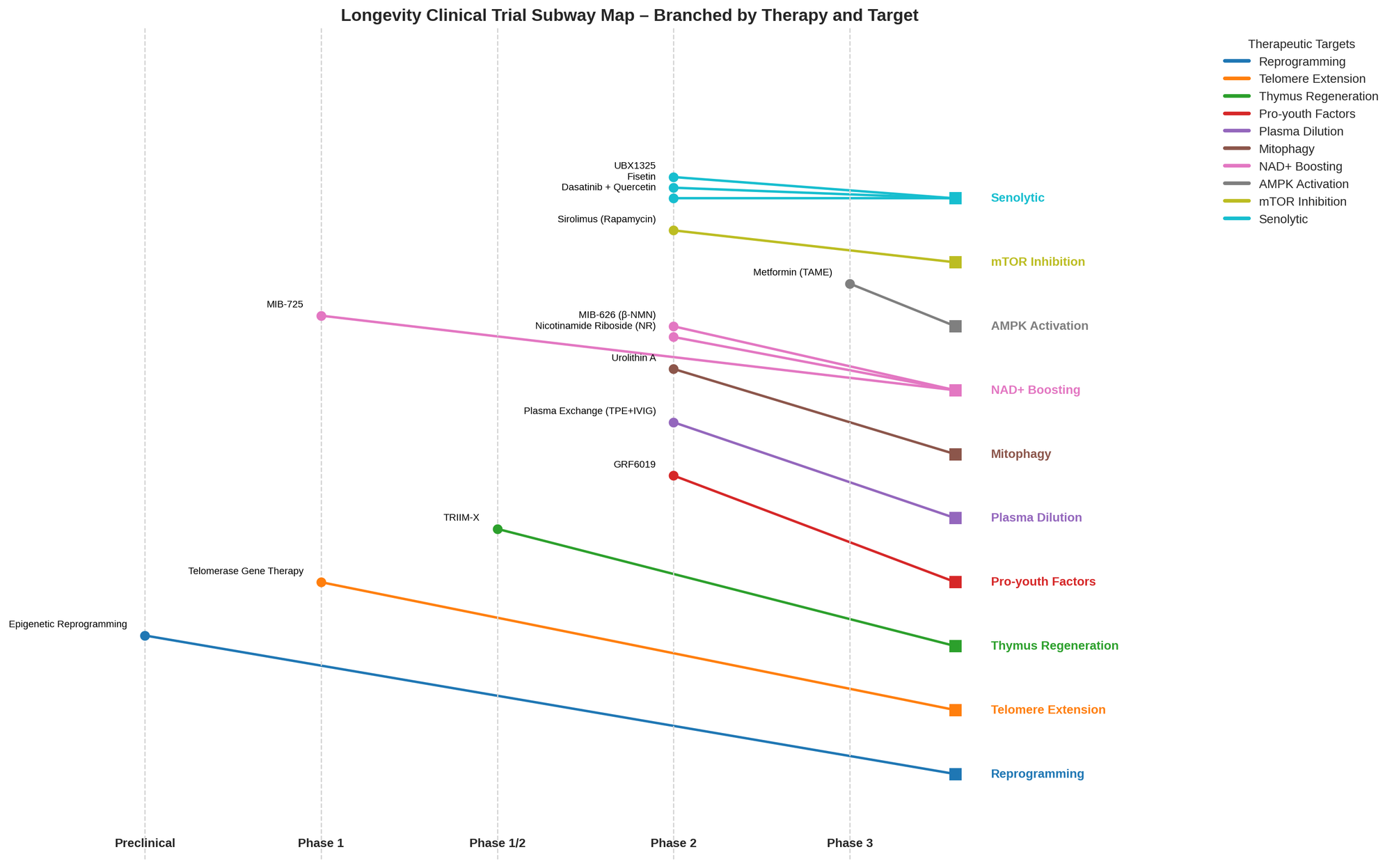
Figure 2: Frequency of therapies by target mechanism in current human trials. Senolytic and NAD-boosting approaches dominate, followed by metabolic modulators, plasma-based therapies, etc., as derived from the list below.
Senolytic Therapies (Clearing “Zombie” Cells)
Dasatinib + Quercetin (D+Q) – Senolytic combo (senescent cell clearance), Phase 2 completed.
This combination of a cancer drug (dasatinib) and a flavonoid (quercetin) was the first senolytic tested in humans. In 2024, Mayo Clinic researchers published a 20-week Phase 2 RCT in 60 women (ages 65–80) showing intermittent D+Q improved markers of bone formation in those with high baseline senescent cell burden (network.mayoclinic.org). The treatment (100 mg D + 1000 mg Q, two days every 2 weeks) was safe and produced modest benefits in bone density at the wrist. Earlier pilot studies (Phase 1) in idiopathic pulmonary fibrosis and diabetic kidney disease had suggested D+Q can reduce senescent cells in humans (sciencedirect.com).
Next steps: D+Q is not yet in Phase 3; further trials are needed to identify optimal dosing and target populations (e.g. people with high senescent cell loads). Notably, Mayo is also running a small pilot (“ALSENLITE”) to see if D+Q penetrates the brain in Alzheimer’s patients (clinicaltrials.gov). No formal clinical trial number is available for the completed bone study (published in Nature Medicine); it was NIH-funded and registered as NCT04313634.
Fisetin – Senolytic polyphenol, Phase 2 ongoing.
Fisetin, a plant-derived flavonoid, showed senolytic effects in mice and is now in a large NIH-funded trial to see if it can help older patients with sepsis. The Senolytics To slOw Progression of Sepsis (STOP-Sepsis) trial is a Phase 2, adaptive, placebo-controlled study (target 100–300 participants) at multiple U.S. hospitals (advances.umn.edu).
Its goal is to determine if fisetin (in a high dose regimen) can reduce inflammation and prevent severe outcomes in sepsis by clearing senescent immune cells. This trial uses an adaptive design to find the optimal dose and efficacy in one go.
Status: Ongoing (as of late 2024) – interim analyses will adjust the trial as data emerges. Fisetin has already shown safety in human pilot studies, and researchers hope it will mitigate the “cytokine storm” and organ damage in sepsis. Trial registration: NCT04771611 (multi-center, led by Univ. of Minnesota and Mayo Clinic).
UBX1325 (Unity Biotechnology) – Senolytic (Bcl-xL inhibitor) for retinal disease, Phase 2.
UBX1325 is an experimental intravitreal drug clearing senescent cells in the retina. It’s being tested for diabetic macular edema (DME) and wet age-related macular degeneration (AMD). Unity Biotechnology reported positive Phase 2 results in DME: in the 24-week BEHOLD study (N=65), a single injection of UBX1325 led to sustained vision gains (+6.2 letters vs –1.3 letters in sham at 24 weeks).
Moreover, the larger Phase 2b ASPIRE trial (N=52) comparing UBX1325 to standard anti-VEGF (aflibercept) found UBX1325 maintained ~+5 letter improvement at 24 and 36 weeks, which was non-inferior to aflibercept in difficult-to-treat patients (globenewswire.com). (It narrowly missed a primary endpoint of strict non-inferiority at weeks 20–24, achieving 88% CI vs the 90% target.) Crucially, UBX1325’s effects persisted longer, suggesting a durability advantage.
Safety: No significant ocular inflammation or vasculitis observed across studies (globenewswire.com).
Unity plans to advance UBX1325 to Phase 3 in DME in 2025. Next readout: Full 48-week data from Phase 2b (ASPIRE) is expected by mid-2025. Trials in wet AMD are ongoing in the US/EU. (ClinicalTrials.gov IDs: NCT04857996 for BEHOLD; NCT05454602 for wet AMD; NCT06011798 for ASPIRE Phase 2b.)
Metabolic Modulators (Targeting Nutrient-Sensing Pathways)
Sirolimus (Rapamycin) – mTORC1 inhibitor, Phase 2 pilot completed.
Rapamycin, an mTOR inhibitor, is a immunosuppressant that extends lifespan in animals. The first randomized trial in healthy aging – the PEARL study (Participatory Evaluation of Aging with Rapamycin for Longevity) – was completed in 2023. In this Phase 2-equivalent trial, 114 adults (aged ~50–60) were randomized to placebo or weekly rapamycin (either 5 mg or 10 mg) for 1 year (lifespan.io).
Results: Rapamycin was generally safe at these low doses, with no significant difference in serious adverse events vs placebo. The primary endpoint (change in visceral body fat) did not differ significantly between groups. However, sex-specific effects emerged: women on rapamycin showed improvements in some blood biomarkers of aging (e.g. slight reduction in biological age measures), whereas men had minor adverse metabolic changes (e.g. slight rise in blood glucose in the 5 mg group).
This aligns with some animal data that females respond more to rapamycin. The trial confirmed rapamycin can slow immune aging (prior shorter studies showed improved vaccine responses (pmc.ncbi.nlm.nih.gov), but did not demonstrate overt functional benefits in a year.
Next steps: Researchers suggest higher doses or longer studies may be needed to see clinically meaningful effects. No Phase 3 is yet planned specifically for “aging,” but smaller trials in specific age-related conditions (e.g. cognitive aging, insulin resistance) are underway. PEARL Trial registration: NCT04488601. Results reported in Aging-US, April 2025 (lifespan.io).
Metformin (TAME Trial) – AMPK activator and metabolic modulator, Phase 3-sized trial initiating.
Metformin, a safe generic drug for type 2 diabetes, is at the center of the landmark Targeting Aging with Metformin (TAME) trial. After years of planning, this multi-center trial is set to start in late 2024, aiming to enroll ~3,000 older adults (65–79) across 14 U.S. research centers (afar.org). The trial will treat half the participants with metformin and half with placebo, and follow them for ~6 years, tracking the onset of any major age-related diseases (cardiovascular events, cancer, dementia, etc.).
The goal is to see if metformin can delay the development or progression of chronic diseases by targeting fundamental aging pathways – effectively, to prove aging itself can be treated. If successful, TAME could pave the way for the FDA to consider “aging” as an indicatable condition.
Status: As of April 2024, TAME had IRB approval and was finalizing funding (a mix of NIH and private sources). Recruitment is expected to begin in 2024. Next readout: Due to its length, interim results may not emerge for several years; the primary completion (end of 6-year follow-up) would be around 2030. (Notably, a smaller parallel trial, VA-IMPACT, is testing metformin in 1,500 veterans; but TAME is the flagship study. No single NCT yet – it’s described as a “study of studies” at multiple sites.)
NAD⁺ Boosters (Enhancing Cellular Energy Metabolism)
Nicotinamide Riboside (NR) – NAD⁺ precursor (vitamin B3 analog), Phase 2 pilot completed.
NR (brand name Niagen®) is an oral supplement that raises NAD⁺ levels, which decline with age. A Phase 2 pilot trial funded by the NIH and ChromaDex tested NR in older adults with mild cognitive impairment. In this randomized placebo-controlled study (20 subjects), NR was escalated to 1,000 mg/day over 8 weeks. It successfully boosted blood NAD⁺ ~2.5-fold compared to placebo and was well tolerated with no serious adverse events (pubmed.ncbi.nlm.nih.gov).
However, cognitive function (MoCA scores) and most other clinical endpoints did not significantly improve over the short duration. There was a hint that NR might reduce brain perfusion deficits (it lowered cerebral blood flow in an overactive brain network, the default mode network), and epigenetic aging in blood slightly improved in NR group (PhenoAge clock), but these results were exploratory. The trial demonstrated safety and target engagement (NAD⁺ up), but no clear clinical benefit in 2 months (pubmed.ncbi.nlm.nih.gov).
Next steps: Longer trials are needed. NR is being studied in other contexts (e.g. muscle aging and inflammationscience.org). Given it’s a supplement, large Phase 3 trials may require government or philanthropic sponsorship. (Trial info: NCT02942888; results published in Geroscience, Feb 2024.)
MIB-626 (β-NMN) – NAD⁺ precursor (NMN analog), Phase 2 ongoing.
MIB-626 is a proprietary, pharmaceutical-grade form of NMN (NAD⁺ precursor) developed by Metro International Biotech (MetroBiotech). In a Phase 1 trial at Brigham & Women’s Hospital, MIB-626 (taken once or twice daily for 14 days) significantly increased NAD⁺ levels in middle-aged and older adults and was well tolerated with no serious side effects (longevitybox.co.uk).
This paved the way for multiple Phase 2 trials that are now underway (as of 2024). Notably, one Phase 2a trial in COVID-19 patients with acute kidney injury is testing if MIB-626 can prevent kidney damage and inflammatory “storm” (NCT05038488). Another Phase 2 is examining short-term MIB-626 in adults with Friedreich’s ataxia (a rare premature-aging disease affecting mitochondria), primarily assessing safety and biomarkers (NCT04817111).
An Alzheimer’s disease trial is also planned (pre-clinical work suggested NAD⁺ boosters might help neuron survival). The COVID/AKI trial is enrolling ~60 patients (placebo vs MIB-626) at Harvard-affiliated hospitals (fdaaa.trialstracker.net), and the ataxia trial is small (≈10 patients). Next readouts: Interim data from these Phase 2a studies could come in 2025.
If results show even modest efficacy (e.g. slowing AKI progression or functional gains in ataxia), MetroBiotech would likely move to larger Phase 3 trials. MIB-626 is also being considered by the U.S. Special Forces for enhancing soldier performance (indicating significant interest in its safety).
All trials are under FDA INDs (biospace.com), highlighting a commitment to pharmaceutical-grade development in contrast to over-the-counter supplement use of NR/NMN.
MIB-725 – Next-generation NAD⁺ analog, Phase 1a initiated.
MIB-725 is a novel NAD⁺ precursor molecule from MetroBiotech, optimized for certain aging-related diseases (e.g. muscle or kidney disorders). A Phase 1a single-ascending-dose trial in healthy adults began dosing in April 2025. In the first cohort (4 of 8 planned participants), no dose-limiting toxicities were observed.
The trial will gradually escalate doses to characterize safety, pharmacokinetics, and NAD⁺ metabolite changes. If a safe dose is identified, a quick follow-on Phase 1b (multiple-dose) will launch at the same Boston site. Next readout: By late 2025, we expect initial safety data from Phase 1a. MIB-725 is MetroBiotech’s second NAD booster in the clinic (after MIB-626) and part of a broader NAD drug pipeline targeting diseases of aging like chronic kidney disease (biospace.com). Trial registry: NCT06815991 (Massachusetts General Hospital).
Mitochondrial and Cellular Health Boosters
Urolithin A (Mitopure) – Mitophagy activator (postbiotic), Phase 2 completed.
Urolithin A is a natural metabolite (derived from pomegranate compounds by gut microbes) that stimulates mitophagy – the clean-up and regeneration of mitochondria. Swiss biotech Amazentis developed a purified Urolithin A supplement (Mitopure) and tested it in a randomized Phase 2 trial on muscle health. In this trial, 66 sedentary older adults (age 65–90) received either 1,000 mg Mitopure or placebo daily for 4 months. Results: At 2 months, the Mitopure group showed significantly improved muscle endurance in the leg and hand (able to sustain muscle contractions longer) compared to placebo (biospace.com).
By 4 months the endurance trend continued, though the 4-month between-group difference was not statistically significant (possibly due to continued improvement in the placebo group as well). A 6-minute walk test improved from baseline in the Mitopure group (mean +25.8 m) while declining slightly in placebo (–21.8 m), but this difference did not reach significance. Importantly, Mitopure was safe and well-tolerated with no serious adverse events.
Interpretation: Urolithin A may counteract age-related muscle fatigue by boosting mitochondrial function (pubmed.ncbi.nlm.nih.gov). The trial’s positive results were published in JAMA Network Open (2022).
Next steps: Urolithin A is already available as a supplement (in Nestlé’s Celltrient® and Timeline® products), but further trials are planned, including a European study on frailty (Amazentis announced a forthcoming EU trial site) and possibly studies combining Urolithin A with exercise.
(ClinicalTrials.gov: NCT03283462; “ENERGIZE” trial at University of Washington.)
Blood-Factor and Plasma Therapies
Plasma Exchange (TPE + Albumin ± IVIG) – “Rejuvenation” via dilution of plasma, Phase 2 completed.
Building on parabiosis research, scientists are testing whether removing “old” plasma factors can rejuvenate tissues. A placebo-controlled Phase 2 trial published in 2024 (Aging Cell) evaluated Therapeutic Plasma Exchange (TPE) in 44 older adults over 6 months. There were four arms: (1) TPE once-weekly for 6 months, (2) TPE twice-weekly for 3 months, (3) TPE + IVIG (intravenous immunoglobulin) twice-weekly for 3 months, and (4) sham placebo infusions. All groups were monitored for changes in biological age biomarkers using 36 epigenetic clocks and multi-omics measures (lifespan.io).
Findings: The TPE+IVIG group showed the strongest signal – after ~1 month of treatment, their composite biological age was 2.61 years lower than baseline on average (vs ~1.32 years lower in the TPE-only group). In contrast, the placebo group’s biological age metrics worsened over time. However, by the end of the trial the rejuvenation effects diminished (“dampened with time” as the body potentially compensated).
Additionally, TPE+IVIG led to rejuvenation of immune cell profiles – treated individuals had T-cell, NK-cell, and monocyte distributions shifting to more youthful patterns. All interventions were safe (no major adverse events reported). Implications: Removing and replacing plasma (with saline/albumin, and replacing beneficial antibodies via IVIG) can transiently reset aging biomarkers. It’s essentially the opposite of young-plasma transfusions – instead of adding factors, it subtracts pro-aging factors.
Next steps: A larger trial is needed to see if these biomarker changes translate into clinical benefits (e.g. improved physical or cognitive function). Also, since IVIG (which provides young antibodies) seemed to enhance the effect, a future arm with IVIG alone would help isolate its contribution.
(This trial, sometimes dubbed “Plasma Dilution” study, was led by researchers from the Stanford/Buck Institute and utilized the Institute on Aging for participant recruitment. No NCT posted; results DOI: 10.1111/acel.13928.)
“Young Plasma” Fraction (GRF6019) – Plasma-derived protein cocktail, Phase 2 completed.
Instead of removing old plasma, another approach is infusing young factors. GRF6019 is a proprietary fraction of young donor plasma developed by Alkahest (now part of Grifols). Two Phase 2 trials have tested GRF6019 in Alzheimer’s disease (AD). In a 2019 trial, 40 patients with mild-to-moderate AD received 5 days of GRF6019 infusions at weeks 1 and 13 (either 100 mL or 250 mL per dose; no placebo group). After 6 months, treated patients showed no decline in cognitive or functional scores (ADAS-Cog, MMSE, ADL) on average (neurologylive.com).
While there was no placebo for comparison, AD patients would typically worsen over 6 months – so maintaining stability suggests a potential slowing of disease. GRF6019 was safe and well-tolerated. A separate Phase 2 in severe AD (N=24, NCT03765762) also found GRF6019 infusions feasible and safe, with hints of reduced inflammation, though patients in that study continued to decline (as expected in late-stage AD). Interpretation: GRF6019 contains a broad mix of plasma proteins; animal studies showed it can rejuvenate the brain (journals.sagepub.com).
The human data are encouraging but inconclusive – the mild AD trial’s lack of decline (without a placebo) needs confirmation in a placebo-controlled study. Next steps: Alkahest/Grifols signaled interest in a Phase 3 trial in Europe (planning was noted when Phase 2 was announced) and possibly combining GRF6019 with conventional therapies.
As of 2025, no Phase 3 outcome has been reported yet (neurologylive.com), so approval is still several years away at best. (Press release: Alkahest, Aug 2019; mild AD trial registration NCT03520998. GRF6019 is also being considered for other neurodegenerative or frailty indications.)
Regenerative and Genetic Interventions
Thymus Rejuvenation (TRIIM-X) – Hormone + drug combination, Phase 1/2 ongoing.
The TRIIM trial (Thymus Regeneration, Immunorestoration and Insulin Mitigation) made headlines in 2019 as the first report of biological age reversal in humans. In that pilot study, 9 men (51–65 years) took a cocktail of growth hormone, DHEA, and metformin for 1 year. The results (published in Aging Cell) showed thymus gland regeneration on MRI, improved immune cell counts, and an epigenetic aging reversal of ~2.5 years on Horvath’s DNA methylation clock (purformhealth.com). Building on this, Intervene Immune (led by Dr. Greg Fahy) launched an expanded trial called TRIIM-X, aiming to include dozens of participants of different ages and optimize the therapy.
TRIIM-X is a personalized protocol – for example, adjusting doses of HGH or adding other immunomodulators – to see if greater or more widespread rejuvenation is possible.
Status: TRIIM-X began around 2020 and is ongoing as a Phase 1/2 study (no results published as of mid-2025). An update in early 2024 indicated the trial had completed treatment in a first cohort by late 2022 and was analyzing data.
The focus remains on immune aging markers (T-cell subsets, thymus fat, infection rates) and general epigenetic aging. Next steps: If TRIIM-X shows significant functional benefits (e.g. fewer infections, improved vaccine responses in treated individuals), the team may seek to conduct a larger, controlled trial. Given that growth hormone has side effects, part of TRIIM-X’s aim is to find safer regimens. This approach, while still experimental, holds that partial reversal of system-wide aging is feasible; it’s one of the first attempts at rejuvenation in otherwise healthy adults.
(Trial registration: NCT04375657, currently not yet reporting results.)
Telomerase Gene Therapy – Telomere extension via AAV-hTERT, Phase 1 (n=1 case).
One of the boldest (and most controversial) attempts to treat aging is through gene therapy: delivering the gene for telomerase (hTERT) to a person’s cells to lengthen telomeres (the chromosome end-caps that shorten with age).
A startup, Libella Gene Therapeutics, announced in late 2019 the “first in-human aging gene therapy trial” – to be conducted in Colombia to bypass U.S. regulations. They proposed treating paying volunteers (at ~$1 million each) with an AAV9-hTERT gene therapy, under the auspices of an IRB-approved trial for biological aging and for Alzheimer’s disease (clinicaltrialsarena.com).
To date, published information is scarce: Libella reported that one 77-year-old man received the therapy in early 2020 (effectively an N=1 experiment) and that his white blood cell telomere length increased from 6.7 kb to 7.33 kb (approx. 8.7% increase) over the following months (data not independently verified). No serious adverse events were reported in that patient, though gene therapy can carry risks of immune reactions or insertional mutagenesis.
The Alzheimer’s-focused arm (seeking to test hTERT gene therapy in AD patients) does not appear to have enrolled anyone as of 2025. Outlook: This gene therapy has not undergone traditional Phase 1 toxicity testing in a stepwise fashion; it’s offered as an experimental service. The approach is very far from approval – it would require convincing evidence of safety and that telomerase activation actually improves healthspan (and doesn’t increase cancer risk). So far, we have only anecdotal results. Mainstream researchers urge caution: while extending telomeres might rejuvenate cells, uncontrolled telomerase expression could promote cancer if tumor-suppressor mechanisms fail.
Next steps: We await any publication from Libella’s trial, but none has been forthcoming. Meanwhile, other groups (e.g. Turn Biotechnologies) are exploring more targeted mRNA or transient gene therapy for telomeres, still in preclinical stages. In summary, the telomerase gene therapy by Libella is an intriguing case study but remains an N=1 anecdote at this point, not an established therapy (clinicaltrialsarena.com).
(Trial registry for Libella’s Alzheimer’s attempt: NCT041 Chrysalis, though it’s not listed in ClinicalTrials.gov anymore; the aging trial was private/IRB-approved only.)
Epigenetic Reprogramming – Partial cell reprogramming (Yamanaka factors), Preclinical (human trials planned).
One of the most exciting frontiers in anti-aging research is partial reprogramming – using transient expression of Yamanaka factors (OSK or OSKM: Oct4, Sox2, Klf4 ± c-Myc) to reset epigenetic marks of aging without erasing cell identity. In mice, this approach has reversed vision loss and improved tissue regeneration, essentially turning back the biological clock in old cells.
Multiple well-funded companies (Altos Labs, Retro Biosciences, Life Biosciences, etc.) are pursuing this, but so far no human trials had begun as of mid-2024 (all are in preclinical development). However, in late 2024 Life Biosciences announced a breakthrough: it plans to launch the world’s first trial of partial reprogramming in humans in 2025.
The initial target is an eye disease – specifically, acute glaucoma or NAION (an optic nerve stroke). The therapy, dubbed ER-100, uses a gene therapy to deliver OSK factors to retinal ganglion cells, activated by a doxycycline switch (ophthalmologytimes.com). In October 2024, Life Bio presented primate data showing a single intravitreal injection of ER-100 preserved visual function and axonal density after an induced optic nerve injury.
Treated monkeys retained much better retinal responses than controls, essentially demonstrating reversal of an age-related vision loss model.
Timeline: The company is on track to begin a Phase 1 trial in optic neuropathy patients in H2 2025. This trial will primarily assess safety (watching for any signs of abnormal growths or loss of retinal cell identity) and secondarily look at vision improvements. If successful, it would be a proof-of-concept that epigenetic rejuvenation can be done safely in humans. Altos Labs and others are not far behind – they are working on tissue-specific partial reprogramming therapies (for example, muscle or liver rejuvenation) but will likely also start with localized, non-systemic indications for safety.
Challenges: By design, OSK gene therapy pushes cells towards a younger state, but too much reprogramming could cause cancer or dedifferentiate cells completely. So the dosing and gene control are critical (altoslabs.com). Nonetheless, this area holds huge promise: in theory, it addresses the root epigenetic drivers of aging. We may see multiple epigenetic reprogramming trials by 2025–2026 (Life Bio’s glaucoma trial, possibly another in muscle or skin from a different company). These are preemptive efforts – human safety data will dictate how fast this moves toward general anti-aging uses. (No clinical trial registry # yet for Life Bio’s ER-100 as of Oct 2024; company statements indicate IND filing in 2025.)
The Role of Supplements in Longevity – A Critical Assessment
The popularity of “longevity supplements” has exploded, with many middle-aged and older adults self-prescribing cocktails of vitamins and plant extracts in hopes of extending life. However, scientific credibility varies wildly among these supplements. Here we focus only on supplements that have some human evidence for impact on aging or healthspan, and we heed expert warnings about overhyping these products.
Firstly, it must be stated: no supplement to date has shown clear evidence of extending human lifespan. Unlike drugs in controlled trials, most supplements lack rigorous studies in aging. Some have promising human data on intermediate outcomes (e.g. improving a risk factor or biomarker), but translating that to longer life or sustained healthspan is unproven.
That said, a handful of compounds stand out as more credible in the longevity context:
NAD⁺ Precursors (NR and NMN)
These are often sold as supplements (e.g. NR as “Niagen” and NMN in various brands). As reviewed above, NR and NMN reliably raise NAD⁺ levels in humans, which is encouraging since NAD⁺ supports cellular energy and DNA repair. Small trials suggest potential benefits: one RCT of NR in older men showed lowered blood pressure and arterial stiffness (2018), and another found NR reduced some inflammatory markers in obese adults. However, no trial has demonstrated that NAD boosters improve hard clinical endpoints in humans yet. In fact, the recent MCI trial saw no cognitive improvement despite NAD⁺ elevation. Regulators are scrutinizing these supplements – the FDA ruled in late 2022 that NMN cannot be sold as a supplement in the U.S. because it’s being investigated as a drug. Bottom line: NAD precursors are biologically active and scientifically plausible geroprotectors (with multiple human trials ongoing), but consumers should temper expectations until larger studies show actual health benefits.
Metformin
Though a prescription drug, metformin is sometimes taken “off-label” by healthy people for longevity. It’s not a supplement per se, but given its wide availability and relatively good safety profile (in non-diabetics it can cause GI upset and B12 deficiency, but serious risks are low aside from those with kidney issues), metformin is one of the most credible longevity medications. Its human evidence is still indirect – observational studies indicate metformin-treated diabetics live longer than diabetics on other therapies, and even possibly longer than some non-diabetics. Small trials in non-diabetics have shown metformin can improve metabolic and inflammatory markers, though notably one trial found metformin blunted muscle gains from exercise in older adults (raising caution about usage in athletic aging adults). The definitive evidence will come from the TAME trial in ~5–6 years. Until then, some longevity physicians do give low-dose metformin to patients with prediabetes or other aging-related indications, weighing its decades-long safety record against its theoretical anti-aging benefits. It’s evidence-informed but not evidence-proven for extending healthspan.
Senolytic Supplements (Fisetin & Quercetin)
Fisetin (a flavonoid from fruits) and quercetin (from onions, etc.) are available over-the-counter and touted as senolytics. Preclinical data on fisetin is strong – it extends median lifespan in old mice and reduces senescent cells. In humans, Mayo Clinic ran a pilot where older women with osteoarthritis took fisetin for 2 days and saw decreased circulating senescence biomarkers. Fisetin is being tested in the aforementioned sepsis trial and a frailty trial. Quercetin alone has poor bioavailability, but combined with dasatinib it has senolytic effects in mice. Some biohackers take “D+Q” or fisetin in intermittent doses (e.g. 2 days a month) hoping to clear senescent cells. The credible part: Animal evidence and initial human biomarker results suggest these supplements may reduce senescent cell burden. The caution: Doses used in trials are high; purity and absorption matter; and we lack data on long-term efficacy or safety in healthy people. Experts urge waiting for ongoing trial results before routinely using senolytics. At this stage, fisetin and quercetin are promising but unproven – they should be viewed as experimental.
Spermidine
Spermidine is a natural polyamine found in foods (like fermented wheat germ). Epidemiological studies link higher dietary spermidine to reduced mortality, and mechanistically it induces autophagy (cellular self-cleansing). A German trial (SmartAge) in 2022 tested a spermidine supplement in older adults with subjective cognitive decline. Findings were mixed: a pilot suggested memory improvements in the spermidine group, but a larger 12-month trial showed no significant benefit on memory, only a non-significant trend of less decline. The interpretation is that spermidine might help preserve cognitive function rather than boost it. It’s considered safe (it’s a component of diet) and is scientifically plausible, so some longevity specialists consider adding spermidine (1–2 mg/day) for older adults at risk of dementia. Still, it joins the theme – some evidence of benefit, but not yet definitive.
Omega-3 Fatty Acids (Fish Oil) and Vitamin D
These standard supplements are not always pitched as “longevity” pills, yet they have arguably stronger evidence for health benefits than many exotic supplements. A large 2018 trial (VITAL) found daily omega-3 (1 g) in 25,000 adults led to a significant 28% reduction in heart attack risk, though no overall mortality reduction. Vitamin D in the same trial did not significantly cut cancer or heart disease incidence, except a suggestion of fewer cancer deaths.
In meta-analyses, vitamin D supplements seem to slightly reduce total mortality in older deficient individuals. While neither is a panacea, maintaining adequate vitamin D and omega-3 levels is a science-backed recommendation for healthy aging (with benefits for bones, heart, and possibly brain). They are “credibly” beneficial for health, if not proven to extend maximum lifespan. Notably, these should be taken in moderation (excessive dosing has no extra benefit and may cause harm – e.g. too much fish oil can increase bleeding risk).
Other Common Supplements
Many other supplements are marketed for longevity with scant human evidence:
- Resveratrol (the red wine polyphenol) gained fame as a sirtuin activator that extended life in obese mice. But human trials have been underwhelming – it’s poorly bioavailable and did not show clear benefits in metabolic or memory studies. It’s largely considered hype now, and high doses could even be risky (e.g. a concern it might stimulate certain cancers).
- Curcumin (turmeric extract) is a potent anti-inflammatory in vitro, but again absorption is an issue and no human trial shows it extends healthspan (it may help arthritic pain or mood in some studies, but results are inconsistent).
- Coenzyme Q10 is an antioxidant often taken by older adults. It can improve heart function in heart failure patients and might slightly boost energy, but for longevity there’s no proof. It also can interfere with common medications (for example, making blood thinners less effective).
- Multivitamins: Interestingly, a recent COSMOS-Mind trial indicated a daily multivitamin might improve cognitive function in older adults over 3 years (the first large evidence that baseline nutrition supplementation could benefit aging brain). However, multivits have not been shown to reduce mortality or cardiovascular events in well-nourished populations. They may help correct micronutrient deficiencies which can otherwise mimic aging-related decline (like B12 for cognition or vitamin D for muscle).
- Alpha-Ketoglutarate (AKG): This Krebs cycle metabolite has been touted as an “anti-aging metabolite.” In 2021, a small non-controlled trial of a calcium-AKG supplement (branded Rejuvant) reported an 8-year reduction in “biological age” as measured by an epigenetic clock, after 7 months of use. While striking, this result in 42 people without a placebo group is hard to interpret – epigenetic age can fluctuate, and the study was funded by the supplement manufacturer. No health outcomes were tracked. AKG is being studied in a proper placebo-controlled trial (by the Mayo Clinic) for effects on inflammatory and frailty markers in older adults, but those results are pending. So, AKG remains intriguing but unconfirmed – it should not be assumed to reverse aging based on the current evidence.
Given the surfeit of supplements, many longevity doctors are now urging restraint. More is not better. Dr. Andrea Maier, a geriatrician and longevity researcher in Singapore, notes that her clinic often has to “de-prescribe” supplements that patients have started on their own. “People think that more helps more, and it’s not the case,” warns Dr. Evelyne Bischof, a longevity physician who has seen patients come in with “a laundry list of longevity supplements” that may do more harm than good. Indeed, piling on dozens of supplements can tax the liver and kidneys and cause dangerous interactions. For example, large doses of NMN might accumulate in the kidneys and cause inflammation, high-dose CoQ10 can interfere with warfarin, and resveratrol might stimulate certain hormone-sensitive cancers. The consensus among experts is to take a personalized, data-driven approach: identify specific deficiencies or risk factors and target those, rather than taking every “anti-aging” pill concurrently.
Longevity specialists often suggest periodic testing to guide supplement use – for instance, check vitamin D levels, omega-3 index, inflammation markers, biologic age measures – and adjust supplements accordingly, rather than blindly following influencer regimens. “If you’re taking a longevity supplement, there’s no evidence that one is good and two is better and three is even better,” as nutrition scientist Naras Lapsys puts it. “A good starting point is to strip down to a lesser number and start measuring”. In practice, that means perhaps focusing on a core few supplements with known benefits (e.g. vitamin D if deficient, omega-3 if diet is poor, maybe a trial of NAD booster or spermidine if one is inclined – but one at a time, measuring effects), and avoiding megadoses.
In summary, supplements in longevity need a critical eye. Some – NAD+ boosters, low-dose aspirin, vitamin D, omega-3 – have scientific rationale and some human data, but even these have not demonstrated an ability to extend lifespan. Many other hyped supplements lack solid evidence and could even be counterproductive. The field is moving toward evidence-driven use of supplements: testing them in clinical trials just as drugs are tested. Until those trials read out, the safest advice is a “less is more” strategy – eat a healthy diet (which provides countless longevity-promoting micronutrients in natural balance), and be judicious with any pills. Supplements are not a magic elixir for aging, and they should complement, not replace, proven health practices like exercise, nutrition, and regular medical care.
Conclusion: Progress and Outlook for Increasing Human Healthspan
As of mid-2025, the field of longevity science stands at an inflection point. The past 12 months have seen an unprecedented number of clinical trials targeting fundamental aging processes rather than individual diseases. This in itself is a major achievement – aging biology is finally being tested in humans in a systematic way. The results so far have been a mix of encouraging signals and humbling reality checks:
We have early evidence that certain strategies do provoke “rejuvenation” at the cellular or molecular level in humans – e.g. senolytics lowering inflammation markers, plasma exchange resetting epigenetic clocks (albeit briefly), NAD boosters restoring a youthful metabolic profile, and mTOR inhibitors potentially preserving muscle mass in some individuals. These proof-of-concept successes validate that human aging is modifiable to a degree.
However, no intervention yet has shown a large, clinical extension of healthspan or lifespan. Many trials reported no significant difference on primary outcomes (e.g. senolytics didn’t improve bone density, rapamycin didn’t cut visceral fat, NR didn’t improve cognition). The improvements seen have been modest or confined to subgroups. It appears that translating dramatic mouse lifespan extensions into humans is far more challenging – humans live much longer than lab animals, and aging is multifactorial and heterogeneous in people.
Safety and feasibility have been a positive takeaway. None of the major trials reported serious adverse effects: senolytic drugs D+Q were well-tolerated even in frail older adults; metformin is known to be relatively safe; NAD precursors showed no safety red flags; rapamycin at low doses did not cause immunosuppression issues over one year. This suggests we can intervene in aging pathways without prohibitive toxicity – an essential condition if preventive use in healthy people is ever to be viable.
The global and multi-phase nature of trials is ramping up. What were once small pilot studies are evolving into Phase 2 and 3 trials with hundreds or thousands of participants (TAME, STOP-Sepsis, MetroBiotech’s trials, etc.). This scale is crucial to detect meaningful effects on hard outcomes (like onset of disease, mortality, or functional decline). The engagement of Big Pharma and biotech investors, alongside academic and government efforts, means resources are flowing into longevity research like never before.
So, where does the field stand on the ultimate goal of increasing human healthspan or lifespan? At present, we have to acknowledge that credible progress is incremental. We do not yet have a proven “age-breaking” pill or procedure. But we do have a much clearer map of the landscape:
Some approaches (e.g. single senolytic agents) might be insufficient alone to markedly improve healthspan, based on current evidence. They might need to be combined (multi-senolytic regimens or senolytics plus rehabilitation) or targeted to specific high-risk groups to show benefits.
Other approaches (like metformin or rapamycin) show potential in specific domains – e.g. preserving immune function or muscle function – but might need optimization (dosing, timing, patient selection). It’s possible their true value will be in certain subpopulations or in combination with other therapies.
Lifestyle and preventative medicine still reign supreme. The backbone of longevity is built on exercise, nutrition, not smoking, sleep, and mental health. Nearly all trials implicitly assume these basics, and indeed some interventions (metformin, rapamycin) may work best in those who have already optimized lifestyle. Interestingly, a recent analysis (2023) suggested that caloric restriction in humans (the CALERIE trial) improved multiple cardiometabolic risk factors and slowed biological aging markers. So, calorie restriction and exercise remain the “gold standards” against which these new therapies are measured. Any pill will ideally mimic some of those effects without the difficulty of sustained lifestyle changes.
The concept of “healthspan” – the number of years of healthy, independent life – is becoming a key endpoint in studies. Rather than just looking at biomarker changes, upcoming Phase 3 trials (like TAME) will examine if people stay disease-free longer. If TAME shows metformin delays multimorbidity, that would be a landmark proof that we can pharmacologically extend healthspan. Even a null result will be hugely informative, guiding the field on what to try next.
Importantly, the past year has underscored the need to not overhype uncertain findings. Longevity science got a lot of media attention (some companies in this space have made bold claims that later had to be tempered). Researchers now emphasize rigorous science and transparency. For example, when the senolytic bone trial showed only a mild effect, the NIA openly highlighted the “potential and limitations” of senolytics, stressing that earlier mouse promise did not fully translate. Such honesty is crucial to maintain credibility.
Looking forward, the field is entering a phase of large-scale testing and refinement. We will likely see, in the next 2–3 years, results from: TAME (metformin), several Phase 2 NAD booster trials, the first large senolytic trials (perhaps in osteoarthritis or lung fibrosis), more data on plasma exchange (with refined protocols), and combination trials (e.g. pairing metformin with exercise, or senolytics with immunotherapy). There’s also increasing interest in biomarker-guided trials – using aging clocks to track efficacy, which could accelerate development by providing early signals rather than waiting for people to develop diseases.
In conclusion, while no breakthrough “fountain of youth” has emerged yet, the credible progress in the last year should not be understated: aging is on the defensive. It is no longer an untouchable inevitability but a biological process we can begin to measure, modulate, and hopefully manage. Each trial – whether yielding positive, null, or mixed results – adds critically to the knowledge of what actually works in humans.
The quest for increased human healthspan will likely be achieved through a combination of strategies, possibly stacking multiple modest interventions to attain a significant overall effect. As of 2025, we are learning which interventions belong in that stack. The optimistic view is that within this decade, we will have the first scientifically validated geroprotective therapy – one that a doctor could prescribe to a middle-aged patient to meaningfully reduce their risk of age-related diseases. The cautious view is that aging is immensely complex, and extending lifespan in healthy humans may require more time and iterative breakthroughs.
What is clear is that the era of credible human anti-aging trials has begun. The field stands well-poised, with robust tools (epigenetic clocks, biomarkers) to evaluate interventions and an expanding pipeline of candidates to test. Each incremental success – be it a drug that safely delays disease by a couple of years, or a therapy that improves an older person’s physical resilience – is a step toward the larger goal of aging better, for longer. The past year’s developments give cause for measured hope: they show progress rooted in science, not science fiction. And as this evidence base grows, the dream of adding healthy years to human life is transitioning from theoretical possibility to practical, clinical reality.
Member discussion