The Geopolitical Royalty: Structuring and De-risking Cross-Border Biotech Revenue Streams Between China and the West

A New Axis of Royalty Flows
China's biotech industry has become an increasingly vital node in global pharmaceutical value chains. Chinese-origin drugs are now out-licensed globally at scale, while Western firms aggressively seek to penetrate China's market via regional deals. These relationships generate complex, multi-jurisdictional royalty agreements, prompting fundamental re-engineering of the standard synthetic royalty model.
As of late 2025, dealflow continues to accelerate. Royalty Pharma has led recent transactions: a $950m deal with BeOne/BeiGene for ex-China rights to Amgen's Imdelltra, and a $75m commitment to Zenas Biopharma's obexelimab program in China.
Cross-border partnerships between Western biotechs and Asian firms have soared in recent years. In 2024, out-licensing deal value for China reached $47 billion (67% CAGR over three years), and by the first quarter of 2025 Chinese companies accounted for 32% of global biotech out-licensing value (up from ~8% in 2021). The first nine months of 2025 alone saw Chinese-origin drug licensing deals exceed $58 billion.
Enforcing Western Biotech IP Rights in China
Western biopharma companies are increasingly able to enforce intellectual property (IP) rights in Chinese courts, with recent cases demonstrating meaningful victories in patent disputes. China's specialized IP courts (established in Beijing, Shanghai, Guangzhou, etc.) have improved fairness and expertise, and foreign patentees now win a majority of cases they bring.
Foreign plaintiffs succeeded in roughly 77% of patent infringement cases against Chinese defendants in 2022 – a win rate comparable to or even exceeding that of domestic firms, reflecting China's efforts to strengthen IP protections.
Recent Landmark Cases (2024-2025)
Recent case examples underscore this trend. Bayer AG won a landmark patent infringement suit in the Beijing IP Court in 2025, securing ¥24.3 million RMB (≈$3.7 million) in damages – the largest patent award in China's life sciences sector to date. The case (Bayer vs. Shenzhen Ante High-Tech) involved a Chinese company infringing Bayer's syringe technology patent, and the court's hefty damages affirmed that Chinese courts will enforce foreign pharma patents even when significant local interests are at stake.
Likewise, Synthes (J&J) prevailed against a Chinese competitor (Double Medical) in 2025, with the Supreme People's Court upholding Synthes' medical device patents and awarding over ¥20 million RMB in compensation. These outcomes – along with wins by companies like Dyson (via mediation) and Levi's (trademark enforcement) – signal that Chinese courts can and do uphold foreign IP rights, from high-tech drug patents to proprietary designs.
Enhanced Legal Remedies
China's patent law amendments (effective 2021) introduced stronger remedies, including punitive damages up to 5× for willful infringement, that further deter infringement and enhance damages awards.
Contract Disputes and Arbitration
While patent enforcement is improving, royalty contract disputes are typically handled via arbitration or litigation under contract law. Chinese courts generally honor well-drafted license agreements, and China's participation in the New York Convention means foreign arbitral awards can be enforced in China barring public policy issues.
For example, if a Chinese licensee failed to pay royalties, a foreign licensor could arbitrate in a neutral forum (Hong Kong is popular for its strong track record of enforcement in mainland China) and then enforce the award in Chinese court. In sum, real-world cases in 2024–2025 show Western biotechs can successfully assert their IP in China, obtaining injunctions and significant damages in patent cases.
The Legal Engine Room: Enforceability and Structural Innovation
Recent precedent indicates enforceability of IP rights by foreign parties is no longer theoretical. Crucially, China is a signatory to the New York Convention, enabling arbitration awards (e.g., via HKIAC or SIAC) to be enforced through PRC courts—unlike foreign court judgments.
Contract Structuring: SPVs and Offshore Escrow
Royalty cash flows must be protected from onshore conversion risks. Standard structuring tools include:
Offshore Payors: Use of a Hong Kong or Singapore SPV as contracting entity, minimizing exposure to SAFE (State Administration of Foreign Exchange) approval cycles.
Parent Guarantees: To buttress an offshore entity with no operating revenue, PRC parents provide a payment guarantee. This must be registered with SAFE to be enforceable.
Escrow Accounts: Contractually mandated escrow accounts (often in Singapore or Hong Kong) serve as neutral cash intermediaries. These accounts disburse quarterly to royalty holders.
NewCo Structures: IP is spun into a Delaware or Cayman SPV with equity allocated to the Chinese licensor. All royalty flows occur offshore, with the Chinese company realizing value via equity.
| Offshore Structure Type | Jurisdiction | Primary Benefit | Key Requirement |
|---|---|---|---|
| Hong Kong SPV | Hong Kong | No capital controls, HKIAC arbitration | Parent guarantee registration |
| Singapore Entity | Singapore | SIAC arbitration, neutral forum | Escrow account setup |
| Cayman/Delaware NewCo | Offshore | Bankruptcy remote, equity value realization | Chinese parent equity stake |
| Escrow Trust Account | Hong Kong/Singapore | Cash quarantine from onshore risks | Independent trustee |
Unique Risks of Non-G7 Royalty Streams
Royalty interests arising from non-G7 markets (like China) pose additional risks compared to purely domestic royalty deals. Key challenges include:
Foreign Exchange (FX) Risk
Revenue in local currency (e.g. CNY) can fluctuate against USD/EUR. Currency controls or devaluation can erode the effective value of royalties. Locking in FX rates via hedges is expensive and must be factored into returns.
Legal Enforcement & Covenant Risk
Enforcing a contract or collecting payments across jurisdictions is harder. Governing law and dispute forums become critical when one party is in China and the other in the West. A standard royalty agreement assumes smooth payment, but here one must plan for "what if the foreign partner doesn't pay?".
Regulatory/Political Risk
Divergent regulatory regimes and geopolitics add uncertainty. China's capital controls can delay or limit cross-border payments. Approvals may be needed for technology transfer or profit repatriation. Political tensions could even impact willingness to honor agreements.
| Risk Category | Description | Impact on IRR | Mitigation Strategy |
|---|---|---|---|
| FX Risk | CNY/USD volatility, devaluation | -2% to -3% annually via hedging costs | NDF forwards, hard currency denomination |
| Legal Enforcement | Contract disputes, payment defaults | Potential total loss | HKIAC/SIAC arbitration, parent guarantees |
| Regulatory Risk | SAFE approvals, capital controls | Payment delays (2-8 weeks) | Offshore SPV, pre-registered contracts |
| Political Risk | Sanctions, expropriation | Variable | Political risk insurance, diversification |
The Financial Core: FX Risk and Internal Rate of Return
Foreign exchange exposure materially alters the IRR profile of a royalty investor. Chinese royalties are often paid in RMB, whereas royalty buyers model in USD or EUR.
1. Currency Denomination
Some deals fix payments in USD, passing FX risk to the Chinese licensee. But this requires approval from SAFE and access to USD liquidity. Others denominate in RMB, shifting the burden to the royalty investor.
2. FX Hedge Cost
Investors often hedge RMB inflows using non-deliverable forwards (NDFs). The cost of a multi-year hedge is a direct function of interest rate differentials:
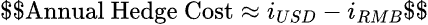
With 2025 U.S. rates at ~5.25% and PBoC rates near 2.50%, hedge costs are ~2.75% annually. On a 7-year horizon, this compounds significantly.
Mid-2025 hedging costs spiked to around 2–3% per annum for many currency pairs, meaning an investor might lose ~2–3% of the value each year by fully hedging RMB over a multi-year period. Over a 5–10 year royalty stream, that could shave tens of millions off returns or require a significantly higher discount rate.
Illustrative Impact of FX Hedge on Royalty Cash Flows
| Year | Unhedged Flow (RMB) | FX Hedge Cost (USD) | Net USD Receipt | Cumulative Impact |
|---|---|---|---|---|
| 1 | 100,000,000 | $2,750,000 | $12,250,000 | -2.75% |
| 2 | 105,000,000 | $2,887,500 | $12,862,500 | -5.61% |
| 3 | 110,250,000 | $3,033,750 | $13,466,250 | -8.58% |
| 4 | 115,762,500 | $3,191,438 | $14,091,562 | -11.66% |
| 5 | 121,550,625 | $3,361,509 | $14,739,141 | -14.85% |
| 6 | 127,628,156 | $3,544,524 | $15,409,792 | -18.14% |
| 7 | 134,009,564 | $3,741,150 | $16,104,806 | -21.54% |
The hedge erodes IRR unless the deal is priced higher upfront:
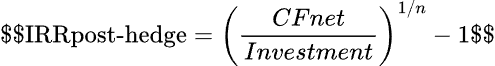
Royalty buyers demand 12–15% IRRs. Hedge costs and repatriation risks push that hurdle rate even higher. As one seasoned investor noted, after accounting for forward hedge costs, the "yield premium" of certain cross-border investments can turn "zero or negative" – in other words, without a risk premium, you'd barely break even versus a risk-free investment once currency is covered.
Navigating SAFE: Repatriating Royalties from China
When Western licensors earn royalties from Chinese sales or licensees, China's State Administration of Foreign Exchange (SAFE) regulations govern how those funds are paid out. Repatriating royalties out of China is feasible but requires a technical, multi-step compliance process to ensure currency conversion and remittance are approved.
Key Steps for Royalty Repatriation
Technology Contract Registration
The royalty-bearing license or technology transfer must be registered with local authorities as a "technology import/export" contract. This involves submitting documentation such as:
- The signed license agreement (with Chinese translation/summary)
- The Chinese licensee's business license and MOFCOM record letter
- The foreign licensor's incorporation certificate
- Details on the technology and payment calculation
For patent or know-how licenses, registration with the local commerce bureau (formerly MOFCOM) is required; for trademark royalties, registration with China's trademark office is needed. Without this registration, Chinese banks can refuse to process royalty wire transfers to the foreign party.
Tax Clearance and Withholding
Royalty payments are subject to Chinese taxes which must be paid and documented prior to remittance. Generally, outbound royalties incur:
- Withholding Income Tax: 10% (a default rate that can be reduced under a tax treaty if the foreign licensor qualifies)
- Value-Added Tax (VAT): 6% (plus surcharges, totaling ~6.72%)
The Chinese licensee acts as the withholding agent, deducting these taxes at the time of payment. The company must file the tax payments with the tax bureau and obtain a tax clearance or record-filing certificate (stamped by the tax authorities) confirming all relevant taxes on the royalty have been paid. This step is crucial, as banks will require proof of tax compliance. If the royalty payment in a single transaction exceeds $50,000 USD, an additional tax bureau filing/approval is required under China's foreign payment rules.
SAFE Filing (if applicable)
In some cases, particularly if the license involves a Chinese guarantor or a performance bond, a filing with SAFE might be needed. For instance, if a Chinese parent company guarantees its offshore subsidiary's royalty payment obligations, that guarantee must be registered with SAFE once a quantifiable amount is determinable. SAFE registration of a cross-border guarantee can be tricky – the agreement should include a covenant that the Chinese guarantor will promptly register it – but it's necessary to ensure any future payout under the guarantee won't be blocked.
Bank Verification and FX Conversion
Finally, the Chinese licensee's bank (authorized for foreign exchange) must review the documents and execute the foreign remittance. The bank will typically ask for:
- The technology import registration certificate
- The tax payment/withholding certificate
- The license contract (or a summary)
- An invoice or payment notice for the royalty amount
Under SAFE's guidance, banks verify the authenticity and compliance of the transaction – essentially confirming the royalties are due under a valid registered contract and all taxes are settled. Once satisfied, the bank will convert the Chinese yuan (RMB) to the foreign currency (e.g. USD or EUR) and remit to the Western licensor's account abroad. This is considered a "current account" transfer (for services/IP fees), which is generally permitted when proper documentation is in place.
PRC Royalty Repatriation Workflow
| Step | Responsible Party | Timeline | Key Documents | Regulatory Body |
|---|---|---|---|---|
| Contract Registration | Licensee | 2–4 weeks | License agreement, business license, tech details | MOFCOM/Commerce Bureau |
| Tax Payment | Licensee | 1–2 weeks | Tax withholding certificate, invoice | State Tax Bureau |
| SAFE Reporting (if needed) | Licensee/Bank | Case-specific | Guarantee agreement, amount determination | SAFE |
| FX Conversion & Transfer | Bank | 1–2 weeks | All above + payment notice | SAFE/Bank |
| Total Estimated Timeline | Various | 4–8 weeks | Complete documentation set | Multiple agencies |
Known Challenges and Timelines
Properly navigating this process can be bureaucratic. Obtaining the technology import registration can take a few weeks, especially if translations or government review are needed. Tax filings for royalties are usually done per payment or quarterly, and getting the tax certificate stamped can add days.
Banks in China tend to scrutinize foreign payments carefully – any inconsistencies (e.g. contract not registered, amounts not matching the contract, or vague invoicing) can delay or block the remittance. It's critical that the paperwork is consistent and complete: even minor discrepancies can trigger requests for additional explanation.
In recent years, SAFE has streamlined some requirements (for example, certain recurrent service payments can use simpler documentation), but companies still report that repatriating funds can take several weeks for each payment cycle in a best-case scenario.
Planning ahead is essential – Western licensors often negotiate clauses requiring Chinese partners to assist in all registrations and to pay any domestic taxes so that the agreed "net" royalty hits the licensor's account. In sum, while China's currency controls do impose an administrative burden, royalties can be repatriated with proper compliance: contract registration, tax payment, and SAFE/bank procedures must all be observed to legally convert and transfer funds abroad.
Deal Structures to Bypass China's Capital Controls
Given China's capital controls and forex regulations, Western biotech licensors and their Chinese partners have developed practical deal structures to facilitate royalty flows and mitigate the risk of trapped payments. These structures aim to either avoid triggering onerous SAFE procedures or provide fallback mechanisms to ensure the foreign party gets paid.
1. Offshore Licensing Entities (Hong Kong/Singapore Conduits)
A common approach is to insert an offshore subsidiary of the Chinese company as the contracting party for the license. For example, a Chinese biotech might have a Hong Kong or Singapore entity (or Cayman Islands holding company) act as the licensee, which then sub-licenses the rights into mainland China to its PRC subsidiary.
Payments are made by the offshore entity to the Western licensor, thus occurring outside of China's FX firewall. This conduit can simplify currency flow since Hong Kong and Singapore have no capital conversion restrictions, and it sidesteps China's onshore payment approvals (the PRC entity may send profits to the offshore affiliate via inter-company agreements or dividends, which is more straightforward in many corporate structures).
However, because the offshore entity is often just a holding company with no assets or revenue of its own, Western licensors typically demand a guarantee from the Chinese parent company (the real operating company) to back the offshore subsidiary's payment obligations. Such a guarantee provides recourse if the offshore SPV fails to pay. Notably, the guarantee itself must be registered with SAFE to be enforceable, as it represents a contingent foreign exchange obligation.
This "license-through-offshore + parent guarantee" model is widely used to ensure royalties can be paid in hard currency and has been explicitly recommended by cross-border transaction advisors.
2. Escrow and Pre-Payment Arrangements
To further alleviate payment risk, some deals utilize escrow accounts or upfront reservoirs of funds outside China. For instance, a Chinese licensee might deposit a chunk of anticipated royalties in an escrow account in Hong Kong at signing, to be released to the foreign licensor upon achievement of sales milestones.
Alternatively, deals may be structured with larger upfront payments and milestone payments (payable upon regulatory approvals or sales thresholds) instead of a high ongoing royalty percentage. Large one-time payments can often be arranged more predictably (even if from China, a single large dividend or outbound transfer can be planned with SAFE) whereas frequent royalty remittances pose repeated compliance efforts.
By converting some of the royalty economics into milestone fees or an "advance" on royalties, parties reduce the number of cross-border transfers. In public deals, outright royalties are sometimes partly monetized via third parties to get cash out: for example, a Chinese company might sell a portion of its royalty rights to an intermediary (often offshore) for a lump sum – effectively externalizing the royalty stream.
3. Offshore SPVs and the "NewCo" Model
A sophisticated trend for China-outbound deals is the NewCo structure, where instead of a direct license, the Chinese company spins off the asset into a new offshore company which receives investment.
In 2024, several landmark Chinese biotech deals adopted this approach. For example, Hengrui Pharmaceuticals granted rights to three drug candidates to a newly formed Delaware company called Hercules (a joint venture funded by foreign VCs) rather than licensing directly to a Big Pharma. Hengrui took a minority equity stake in the NewCo and will receive milestones and royalties on the drugs' future sales.
By conducting the transaction via a U.S. SPV, Hengrui enabled global investors to fund the asset's development without navigating China's outbound investment or FX approval process. Essentially, the IP was moved to an offshore entity at the deal's outset, so any royalties flow outside China (the NewCo pays Hengrui from offshore) and value can later be realized via the NewCo's sale or IPO.
Other Chinese biotechs like Keymed and Genor also executed similar spin-off licensing deals in 2024. This model highlights how using offshore vehicles and joint ventures can bypass many PRC restrictions: the Chinese firm swaps some of its license value for equity in the offshore venture (which is easier than repatriating cash), and foreign capital drives development.
The NewCo strategy is not applicable to every deal (it works best for significant assets that justify a standalone company), but it has become a popular alternative to traditional licensing, precisely because it sidesteps direct cross-border royalty flows while still compensating the Chinese innovator.
| NewCo Deal Examples (2024) | Chinese Company | Offshore Entity | Structure | Deal Value |
|---|---|---|---|---|
| Hercules Biopharma | Hengrui Pharmaceuticals | Delaware Corp | 3 drug candidates, minority equity | $5.7B potential |
| Unnamed NewCo | Keymed Biosciences | US Entity | Asset spin-off with VC funding | Undisclosed |
| Global Development Co | Genor Biopharma | Offshore SPV | IP licensing + equity stake | Undisclosed |
4. Parent Company Guarantees and Credit Enhancements
Where an offshore SPV isn't used, deals often build in other credit support to manage risk. A Chinese licensee that is a subsidiary of a larger group may have its offshore-listed parent or a Hong Kong affiliate serve as a co-obligor or guarantor for payments. This provides the Western licensor a direct claim against an entity that likely holds foreign currency or assets abroad.
Even state-owned pharma companies have provided such guarantees in certain collaborations to assure payment performance. Another tool is negotiating penalty clauses or interest on late payments, to incentivize timely remittance despite capital control hurdles. While these don't remove regulatory barriers, they compensate the licensor for any delays.
In some cases, Western licensors also retain the right to terminate the license or convert it to a non-exclusive license if royalties are not paid within a certain grace period – effectively using the threat of lost exclusivity to ensure the Chinese side prioritizes the outbound transfer.
These legal backstops, combined with creative financial structuring (like routing payments through less restrictive jurisdictions), collectively mitigate the challenges of China's capital controls. Publicly disclosed transactions, such as Hengrui's Delaware JV or BeiGene's use of a Swiss entity (BeOne) to sell royalties, showcase how deals are being structured to either keep the cash flows offshore or provide alternative paths for value transfer.
Managing FX Risk: Hedging and Currency Terms
Foreign exchange risk is one of the most significant (and quantifiable) challenges in monetizing non-G7 revenue streams. A royalty stream in Chinese renminbi (RMB) introduces volatility for an investor whose obligations (or returns) are in USD or EUR.
Hard-Currency Denomination
Wherever possible, cross-border deals denominate payments in a stable currency like USD. Many China licensing contracts stipulate that milestones and royalties are calculated in USD (or converted to USD at a fixed rate or prevailing rate) – thus the Chinese payer bears the FX conversion on their side.
For example, a Chinese licensee might owe "10% of net sales in China, paid in USD." This shifts some currency risk to the Chinese party (if RMB weakens, it costs them more RMB to buy the same USD amount). However, even USD-denominated obligations don't eliminate FX risk entirely: the Chinese company still needs regulatory clearance to purchase USD and remit them abroad. If USD availability is restricted or the RMB depreciates sharply, payments could be delayed or defaulted.
Investor FX Hedges
Royalty investors will often hedge the expected foreign currency inflows using forward contracts or options (e.g. an NDF – non-deliverable forward – for RMB). This locks in an exchange rate for future royalties, converting uncertain RMB flows into predictable USD or EUR.
The downside is cost. In 2025, global interest rate differentials made currency hedging particularly expensive, putting FX risk "at the top of investors' agendas". Hedging cost is roughly equal to the interest rate gap between currencies. For instance, if Chinese interest rates are lower than U.S. rates, an RMB→USD forward will price in RMB appreciation – effectively costing the hedger the difference.
Comparative Hedging Cost Analysis (2025)
| Currency Pair | Interest Rate Differential | Annual Hedging Cost | 5-Year Total Cost | Impact on 12% Target IRR |
|---|---|---|---|---|
| CNY/USD | ~2.75% | 2.75% | 13.75% | IRR reduced to ~9.5% |
| EUR/USD | ~1.25% | 1.25% | 6.25% | IRR reduced to ~10.9% |
| JPY/USD | ~4.50% | 4.50% | 22.5% | IRR reduced to ~8.0% |
| KRW/USD | ~3.00% | 3.00% | 15.0% | IRR reduced to ~9.3% |
Sharing or Capping FX Risk in the Contract
Another way to re-engineer the royalty agreement is to build in FX risk-sharing mechanisms. For example, the royalty rate could step up if the local currency weakens beyond a threshold (to compensate the payee for lost value), or a floor/ceiling exchange rate could be agreed for conversions.
These provisions are complex and less common, as they effectively hedge via contract, but they underscore the focus on FX in cross-border deals. More frequently, the biotech seeking financing simply has to accept a higher cost of capital because the investor will price in a cushion for currency volatility.
Ensuring Enforceability and Mitigating Default
Legal enforceability is another pillar that must be fortified in cross-border royalty agreements. When revenues depend on a counterparty or sales in China, the agreement has to account for what happens if things go wrong.
Governing Law and Forum
Rather than submit to the local jurisdiction of the foreign partner, deals almost always choose a neutral governing law (New York or English law are common) for the royalty contract. Chinese parties generally accept this for cross-border deals; PRC law even explicitly allows foreign law in contracts involving a foreign entity.
However, using New York law on paper is only half the battle – one must also ensure any judgement or award can be enforced. China will not enforce foreign court judgments from the U.S. or most Western countries (there's no bilateral treaty, and only a slim "reciprocity" possibility).
This means if the biotech sued the Chinese counterparty in New York and won, collecting on that judgment in China would be extremely uncertain. Arbitration is therefore the preferred dispute mechanism. China is a signatory to the New York Convention on arbitral awards, so a properly obtained foreign arbitral award "is prima facie enforceable" in China.
Royalty agreements are being written to mandate arbitration (often under HKIAC – Hong Kong International Arbitration Centre, or SIAC in Singapore) for any major disputes. Hong Kong in particular has become a popular forum due to its track record of China enforcement and its ability to grant interim relief like asset freezes in mainland China.
Contractual Remedies and Security
A cross-border royalty contract will include strong covenants and remedies to deter non-payment:
Termination and Reversion: If the commercial partner fails to pay royalties (or breaches other key terms), the licensor often has the right to terminate the license and reclaim the product rights in that territory. The threat of losing their rights to a lucrative drug is a powerful incentive for a licensee to honor payments.
Penalty and Interest Clauses: High interest on late payments, and even pre-agreed liquidated damages for breach, can be stipulated. While collecting such penalties abroad might be tough, they set the tone that non-compliance is costly.
Guarantees or Letters of Credit: As mentioned, a parent company guarantee is often required from the foreign partner's main corporate entity. In some cases, an irrevocable standby letter of credit from an international bank may be requested, which the licensor/investor can draw on if royalties aren't paid.
Escrow of IP or Data: Another creative safeguard is putting certain critical assets in escrow. In China, one concern is that if a Chinese licensor goes bankrupt, the foreign licensee might lose its rights (since China lacks an equivalent to U.S. Bankruptcy Code §365(n) protecting licensees).
| Enforcement Mechanism | Type | Effectiveness | Implementation Complexity | Cost |
|---|---|---|---|---|
| HKIAC Arbitration | Legal | High | Medium | $50K-$500K |
| SIAC Arbitration | Legal | High | Medium | $40K-$400K |
| Parent Guarantee (SAFE-registered) | Financial | Very High | High | Minimal (registration fees) |
| Standby Letter of Credit | Financial | Very High | Low | 1-3% of guaranteed amount |
| IP/Data Escrow | Asset-based | Medium | High | $10K-$50K annually |
| Termination Right | Contractual | Medium | Low | Minimal |
Practical Enforcement Planning
Even with arbitration in place, parties will think through enforcement before problems arise. This means identifying what assets the foreign partner has offshore (e.g. in Hong Kong or U.S. subsidiaries) that could be targeted to satisfy an award.
It also means ensuring the agreement and the transaction comply with all local regulations up front – for example, making sure the license is properly registered with MOFCOM and SAFE in China. If the contract isn't registered as required, Chinese banks might block or delay royalty payments leaving the country.
Royalty Pharma's Strategic China Pivot
Royalty Pharma (the world's largest pharma royalty investor) has recently provided strategic commentary on the burgeoning biotech royalty dealflow involving China, offering a clear window into current trends. At Royalty Pharma's Investor Day in September 2025, management highlighted China as an emerging "megatrend" in their industry and detailed how they plan to capitalize on it.
Explosion of China-related Deals
Royalty Pharma noted the meteoric rise in licensing deals originating from Chinese biotechs. In 2019, essentially zero major out-licensing deals came out of China, whereas by 2022/2023 there were dozens – "last year, 43 deals, and on pace for more this year". By 2024, roughly one-third of all global licensing deals for new therapies involved a China-originated product.
This represents a massive shift in just a few years. The driver is that Chinese biopharma R&D has matured, and many innovative drug candidates from China need global partners. Royalty Pharma sees this as fertile ground: every license from a Chinese company to a Western pharma creates a royalty stream (usually the Chinese company retains a royalty on ex-China sales, or the Western company may pay milestones/royalties).
Early Moves – High-Profile Royalty Deals
In 2025 Royalty Pharma began actively deploying capital into royalties linked to Chinese innovation. Notably, in August 2025 Royalty Pharma agreed to pay up to $950 million to acquire a royalty interest from BeOne Medicines (formerly BeiGene) on Amgen's cancer drug Imdelltra. This deal involved a $885 million upfront payment for ~7% of worldwide sales, with BeOne retaining China sales rights. It showcased Royalty Pharma's ability to structure around China: BeOne (BeiGene) had domiciled the relevant rights in a Swiss entity, enabling a clean purchase.
Another example: in September 2025 Royalty Pharma invested $75 million in Zenas Biopharma for a royalty on the autoimmune drug obexelimab (as part of a $300 million funding agreement). Zenas is developing the drug in China (and globally), and Royalty Pharma's funding is secured by future royalties if the drug succeeds.
These transactions, disclosed in Royalty Pharma's filings, underline that Chinese-origin science is now part of Royalty Pharma's deal pipeline. As CEO Pablo Legorreta explained, the company has spent years building relationships with Chinese biotechs and VCs, positioning itself to broker such deals.
Strategic View – "We Need to Be There"
Royalty Pharma's leadership characterized the China opportunity as both important and inevitable for the royalty business. In the Investor Day Q&A, Legorreta noted the quality of Chinese biopharma innovation is "incredible" and accelerating, with many programs that will be commercialized abroad. He emphasized that Royalty Pharma must participate in this trend: "these companies need partners…that creates royalties. So from our perspective, many more products developed in China will carry royalties. We need to be there, and we will be there".
The company indicated it is dedicating resources to focus on China and 'dominate that market' for royalty financing as it grows. This is a notable strategic shift – essentially an acknowledgement that a significant portion of future royalty deals (and funding needs) will come from Chinese biotechs out-licensing or monetizing assets.
Royalty Pharma expects that as Chinese capital markets face challenges (capital is "more scarce in China"), Chinese innovators will increasingly turn to royalty funding as a non-dilutive financing route. Interestingly, Legorreta also pointed out that royalties have an advantage in China's geopolitical context: royalty investments are not equity stakes, and thus may be "easier to bring capital to [Chinese] companies" without the regulatory and political sensitivities that come with equity ownership by foreign entities.
Expected Dealflow and Outlook
The commentary and data from Royalty Pharma suggest a robust pipeline of China-related deals going forward. The firm cited industry data that 250+ licensing deals occur annually for new therapies globally, and a growing slice of this is from China.
Royalty Pharma's public statements and Q3'25 earnings discussions indicate they foresee multiple opportunities to buy royalties from Chinese firms who have partnered assets with Western pharmas. For instance, whenever a Chinese biotech licenses a drug to a big pharma, Royalty Pharma could later acquire the Chinese company's retained royalty.
| Royalty Pharma China Deals (2025) | Partner | Asset | Deal Value | Structure | Territory |
|---|---|---|---|---|---|
| BeOne/BeiGene-Amgen | BeOne Medicines | Imdelltra (tarlatamab) | $950M (up to) | 7% royalty purchase | Ex-China (worldwide) |
| Zenas Biopharma | Zenas BioPharma | Obexelimab | $75M ($300M total) | Royalty-backed funding | China + Global |
Additionally, Royalty Pharma can offer upfront funding to Chinese biotechs in exchange for a royalty – effectively providing growth capital. The September 2025 Investor Day presentation reinforced that royalties are becoming a mainstream funding paradigm and that Royalty Pharma is uniquely positioned as "partner of choice" to innovators.
With Chinese biotechs now a major source of innovation, we can expect Royalty Pharma to announce more deals like BeOne/BeiGene and Zenas. Indeed, management hinted that China's contribution to their numbers, while minimal so far, "could be big" over the next few years.
Re-engineering the Synthetic Royalty Agreement for Global Risk
In light of the challenges outlined above, a "standard" royalty financing agreement must be substantially re-engineered when the revenue source is a non-G7 market. The goal is to manage legal, currency, and political risks in an integrated way.
1. Offshore Payment Channels & SPVs
Don't rely on onshore cash flow. Structure the deal so that royalties transit through an offshore conduit under neutral jurisdiction. This may involve using a foreign special-purpose vehicle (SPV) or subsidiary as the license holding entity, and requiring all royalty payments to be made to an offshore escrow or trust account.
By keeping the money outside the high-risk country's direct control, you insulate the revenue stream from local banking restrictions and potential government interference. The SPV holding the royalty rights can be made bankruptcy-remote from the biotech, ensuring the investor's claim on those royalties won't get entangled if the biotech hits trouble.
2. Robust Legal Framework (Choice of Law & Enforcement)
Use a internationally trusted governing law (e.g. New York or English law) for contract clarity, but more importantly, build in an enforceable dispute resolution mechanism. The agreement should mandate arbitration in a forum where awards can be enforced against the foreign counterparty (for instance, HKIAC arbitration with seat in Hong Kong, leveraging China's obligation to honor New York Convention awards).
Also obtain any necessary guarantees from parent entities and ensure they are compliant with local law (such as registering a Chinese parent guarantee with SAFE). These steps mean that if a breach occurs, the investor/issuer has a clear, quick path to obtain a remedy and convert it into assets – rather than being stuck with an unenforceable judgment.
3. FX Risk Mitigation Built-In
Incorporate the reality of currency risk into the deal economics. This can mean denominating payments in a hard currency (with the necessary regulatory approvals for conversion) to reduce FX fluctuation. It also means the financing should price in the cost of hedging the foreign currency stream.
For example, if USD/RMB forward hedges cost ~3% per year (based on interest rate differentials), the royalty rate or upfront payment will need to be adjusted so that the investor's net return (after hedging) meets their target IRR. The agreement might allow the royalty payer some flexibility in timing currency conversions (to optimize rates) but not at the expense of the payee's risk.
4. Alignment on Regulatory & Political Contingencies
A cross-border royalty agreement should acknowledge the "sovereign risk" context and include provisions to handle adverse government or political events. This might involve obtaining political risk insurance for extreme cases (e.g. government expropriation of funds, new laws blocking payments) – a cost that could be shared in the deal structure.
It also means the foreign partner must commit to maintain compliance with all local laws that affect the deal: e.g. agreeing to secure foreign exchange approvals, register the contract with authorities, and adhere to any technology import/export rules.
| Re-engineering Element | Implementation | Primary Benefit | Typical Cost |
|---|---|---|---|
| Offshore SPV Structure | HK/Singapore/Cayman entity | Bypasses capital controls | $50K-$200K setup |
| Escrow/Trust Account | Independent trustee in neutral jurisdiction | Cash quarantine and security | 0.5-1% of flow annually |
| HKIAC/SIAC Arbitration Clause | Mandatory arbitration provision | Enforceable awards in China | Minimal (drafting) |
| Parent Guarantee (SAFE-registered) | Chinese parent backs offshore SPV | Payment security | Registration fees |
| Currency Hedging Strategy | NDF forwards, options | Locks in exchange rates | 2-3% annually |
| Political Risk Insurance | Third-party policy | Protection from expropriation | 1-5% of insured amount |
| Hard Currency Denomination | USD/EUR payment terms | Reduces FX volatility | SAFE approval required |
| Enhanced Covenants | Termination, reversion rights | Strengthens enforcement | Minimal (drafting) |
Conclusion
Cross-border royalty deals involving China require hybrid structuring: offshore vehicles, FX hedging overlays, and pre-engineered enforcement mechanics. The legal enforceability of IP rights in China is real but requires proactive jurisdictional planning. Currency volatility and SAFE compliance materially raise capital costs.
Yet as China's pipeline globalizes, biotech royalty flows from—and into—China will only increase. Structuring them correctly will define the next decade of biotech capital markets. The question posed was how to re-tool these agreements for global risk – the answer is through a combination of legal innovation and financial engineering that ensures a royalty from Beijing can be as bankable as one from Boston.
With the right structuring, cross-border royalties can indeed fuel biotech innovation worldwide, without leaving investors hostage to geopolitics. The standard synthetic royalty agreement must evolve into a geopolitically-savvy financing instrument. By re-engineering contract terms and cash flow structures to manage currency conversion, enforceability, and political uncertainties, dealmakers can monetize revenue streams from non-G7 markets both efficiently and safely.
Key Takeaways for Structuring China-West Royalty Deals
- Legal Enforceability is Real: Chinese courts now enforce foreign IP rights with 77% success rates for foreign plaintiffs, landmark damages awards exceeding ¥20M, and arbitration via HKIAC/SIAC provides enforceable remedies.
- Offshore Structures are Essential: Use Hong Kong/Singapore SPVs, escrow accounts, and NewCo models to route payments outside China's capital controls and SAFE approval processes.
- FX Hedging is Expensive but Necessary: Currency hedging costs 2-3% annually, which can reduce target IRRs from 12-15% down to 9-10% over multi-year periods—this must be priced into deals.
- SAFE Compliance Takes Time: Royalty repatriation requires 4-8 weeks minimum with proper documentation: contract registration, tax clearance (10% withholding + 6.72% VAT), and bank verification.
- The NewCo Model Bypasses Many Issues: Spinning IP into Delaware/Cayman entities allows Chinese companies to realize value via offshore equity while foreign investors avoid repetitive SAFE processes.
- Royalty Pharma is Aggressively Entering China: With $1B+ deployed in 2025 (BeOne/BeiGene and Zenas deals), Royalty Pharma sees China as a "megatrend" representing 32% of global out-licensing value.
- Re-engineering Requires Integration: Successful cross-border royalty agreements combine offshore payment channels, HKIAC arbitration clauses, parent guarantees, FX hedges, and political risk provisions into a comprehensive risk management framework.
Disclaimer: This article is for informational purposes only and does not constitute legal, financial, or investment advice. The author is not a lawyer or financial adviser. All information is derived from publicly available sources and may not be complete or current. Details regarding transactions, royalty structures, and financial arrangements may change. Readers should conduct their own due diligence and consult with appropriate legal and financial professionals before making any decisions.
Member discussion