What Are Drug Targets? A Sceptic’s Guide to Molecular Bullseyes
1. Introduction: The Gospel of the Target
In the parlance of modern pharmaceuticals, few words carry the theological weight of "target." Scientists chant it, investors demand it, and regulators inquire after it with the reverence of high priests examining relics. If one believes the marketing, every drug these days is a "targeted therapy," aimed squarely at some culpable molecule like a sniper on a rooftop. But what is a drug target, really? And why is everyone so desperately aiming?
In brief, a drug target is a molecule in the body—typically a protein, sometimes a nucleic acid or a carbohydrate—that, when fiddled with chemically, changes the course of a disease. Think of it as a lock into which your drug is supposed to fit, ideally disarming some biochemical burglar. In practice, it’s more like trying to pick the correct lock in a wall of identikit mailboxes while blindfolded, with gloves on, and while being shouted at by analysts from Morgan Stanley.
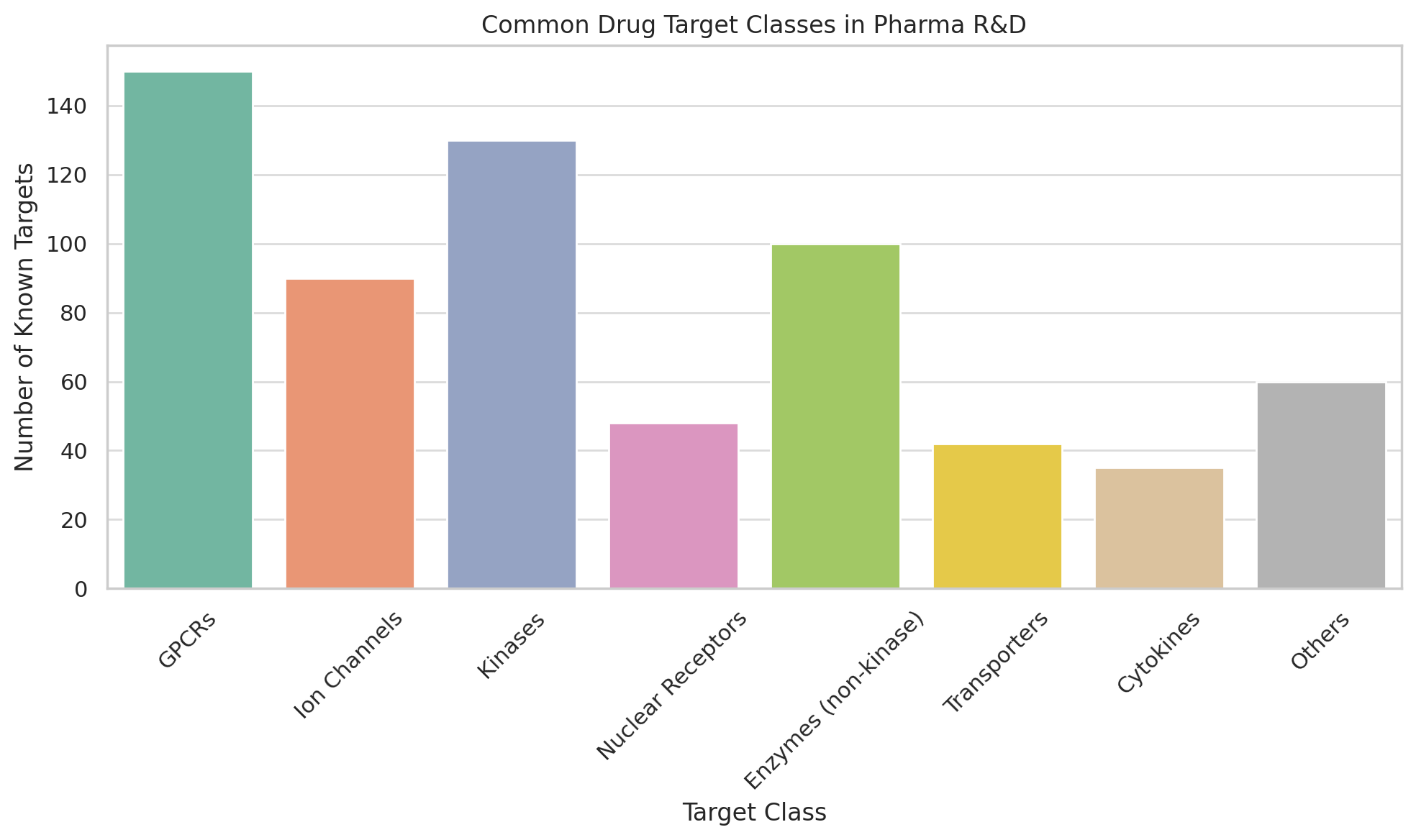
2. Molecular Biology for Grown-Ups: The Target Zoo
The menagerie of drug targets includes:
- Receptors (such as G-protein coupled receptors, or GPCRs): These proteins sit on cell surfaces like bouncers at a nightclub, letting some molecules in and keeping others out. Beta blockers, for instance, owe their fame to blocking beta-adrenergic receptors in the heart.
- Enzymes: The chemical handymen of the cell. Inhibiting them can stop a cascade of unfortunate molecular events. Aspirin, the humble analgesic, inhibits cyclooxygenase—COX—an enzyme responsible for inflammation (and pain, and joyless mornings).
- Ion Channels: Think of these as gates that allow charged atoms (ions) into or out of cells. Drugs that target ion channels often meddle with nerve signals. Lidocaine numbs your tooth by bullying sodium channels into submission.
- Nuclear Hormone Receptors: These are intracellular targets that control gene expression. Estrogen receptors, for example, are the reason tamoxifen exists and why breast cancer sometimes doesn’t.
- Transporters, scaffolding proteins, cytokines, kinases, proteasomes, integrins, Toll-like receptors...
There is a target for every disease, and sometimes several diseases for every target. Whether this is a testament to biological ingenuity or pharmaceutical indecision is up for debate.
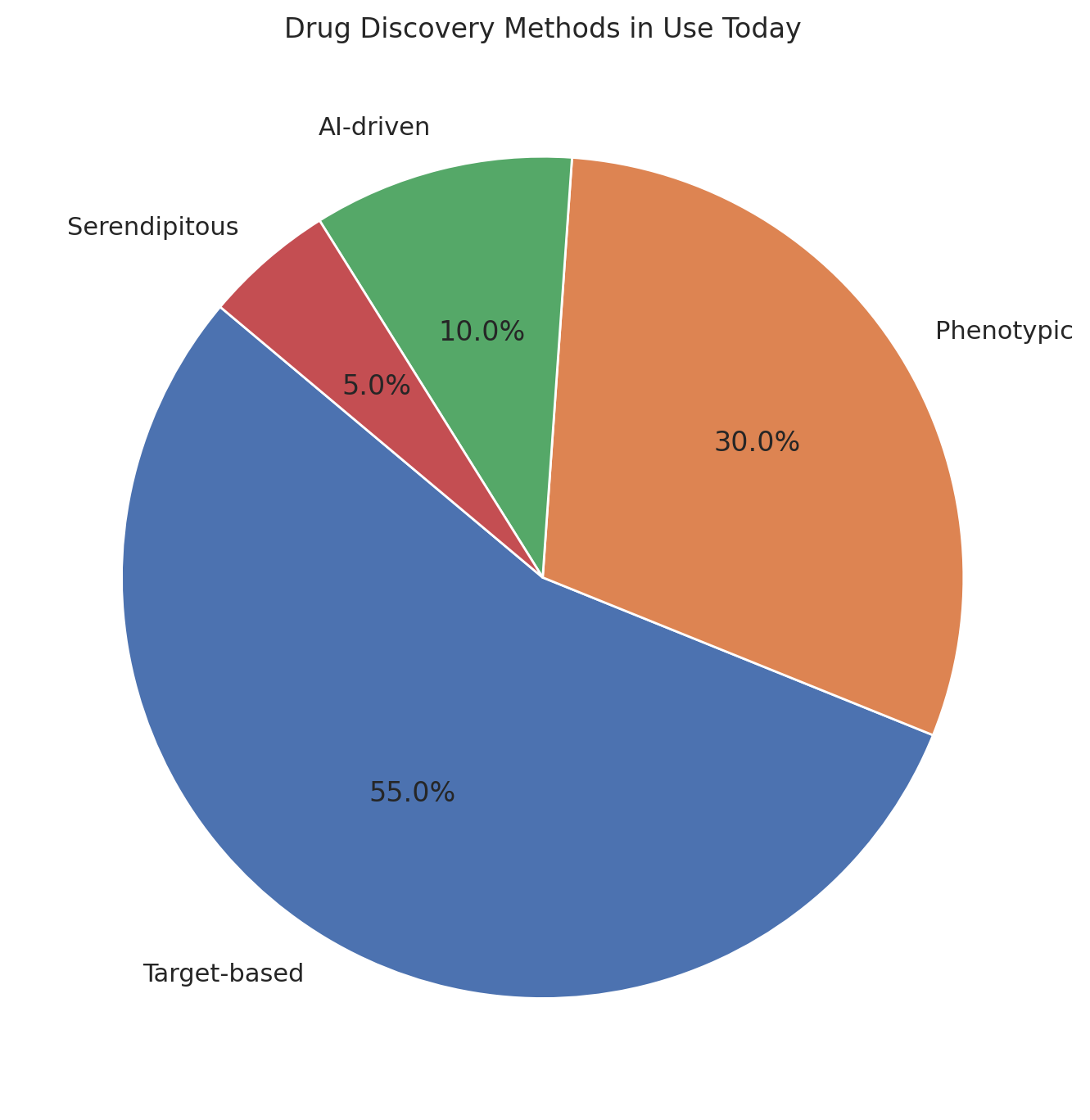
3. How Drug Discovery Really Happens
There are two kinds of people in the drug world: those who start with a target and those who start with a result.
- Target-based drug discovery involves picking a molecule (say, a kinase) and then throwing hundreds of thousands of compounds at it in vitro to see if anything sticks. If it does, great. If it doesn’t, you quietly pivot to something with a less embarrassing failure rate.
- Phenotypic screening, the older and in many ways humbler method, involves exposing cells or organisms to compounds and simply observing what happens. The target, if there is one, can be identified later with the forensic glee of a biochemist with tenure.
The target-based approach looks better in investor slide decks; phenotypic screening looks better in Nobel citations.
4. Fads and Fashions: The Usual Suspects
Drug targets, like hemlines and hedge funds, are subject to fashion.
- HER2: Once a niche receptor in breast cancer, HER2 now appears to be the molecular equivalent of an aspiring actor: in breast, gastric, lung, and perhaps eye cancer, depending on who you ask. It launched several blockbuster ADCs (antibody-drug conjugates), and a thousand press releases.
- TROP2: The poster child for triple-negative breast cancer and other tough-to-treat tumors. If your biotech doesn’t have a TROP2 ADC in Phase 1, do you even biotech?
- KRAS: Once considered “undruggable,” KRAS is the Rasputin of molecular oncology. It refused to die. Miraculously, it’s now drugged—sort of. The G12C mutation can be targeted, but the celebration was cut short when resistance showed up uninvited.
- CD19 and CD20: These B-cell targets made CAR-T therapy possible, and hematologists irrationally exuberant.
- PD-1 / PD-L1: The rockstars of immunotherapy. These molecules prevent your immune system from going berserk—sometimes too effectively. Blocking them unleashes T-cells upon tumors, and sometimes upon anything else that moves. Popular, profitable, and predictably crowded.
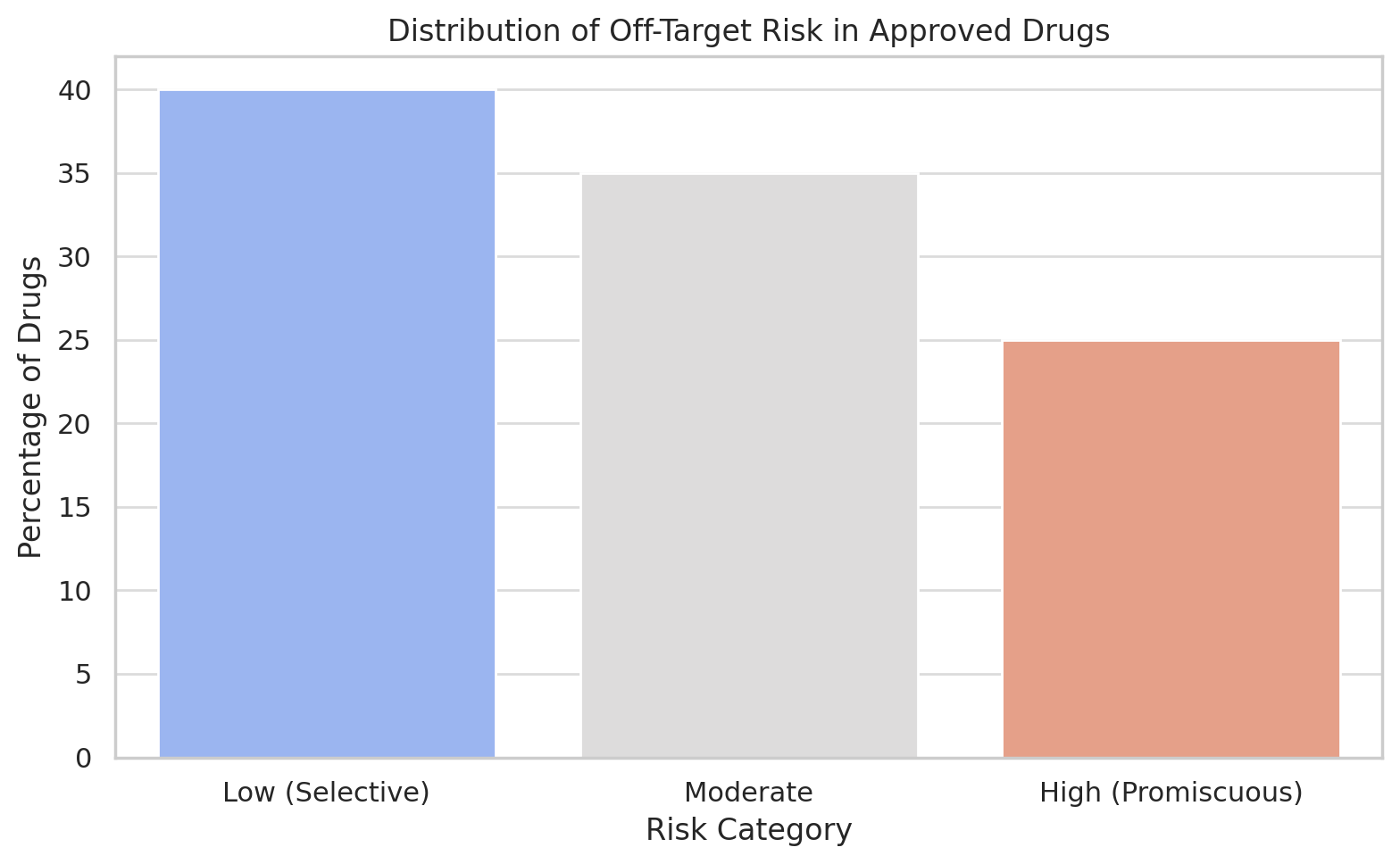
5. The Perils of Promiscuity
Unfortunately, biological systems don’t read journal articles. The drug you designed to hit one protein might also flirt with three others. This is called off-target activity, and it’s the molecular equivalent of sending a love letter to the wrong address.
The consequences range from mildly inconvenient (nausea) to catastrophic (death, bankruptcy, a stern letter from the FDA). Selectivity is a drug’s virtue; promiscuity is its vice. Still, in some cases off-target effects are rebranded as “multi-target synergy,” especially when you can’t explain them but they seem to work.
6. Who Picks the Targets?
Target selection is an opaque process, somewhere between science, art, and PowerPoint.
- Academia: Tends to pick targets based on biological interest and the promise of publications. Whether these targets are druggable or relevant to disease is sometimes a secondary concern.
- Industry: Picks targets based on unmet need, market size, competitive differentiation, and how well they look in a waterfall plot.
- AI & Machine Learning: The new kids on the block. These tools can ingest vast omics datasets and spit out targets that are often intriguing, sometimes actionable, and occasionally hallucinatory. Whether this is progress or pareidolia remains to be seen.
The holy grail is a valid target: one with clear disease linkage, measurable modulation, and a pathway to patentable molecules. It is rarer than it sounds.
7. The Myth of Druggability
Some proteins are famous not for what they do, but for how hard they are to drug. KRAS was in this category until recently; others like MYC remain infamously slippery. These molecules may drive disease, but they have no clear pockets or structures for drugs to latch onto.
This leads to the industry-wide game of trying to either stabilize them, degrade them (e.g., PROTACs), or just pivot to their downstream effectors. It’s a bit like trying to change government policy by convincing someone who once had lunch with a deputy minister.
8. Target Inflation and the AI Pipeline
The introduction of AI has supercharged the generation of new targets—far faster than any wet lab can validate. AI engines now spit out molecular candidates by the thousands, most of which sound impressive (CDx47, HNMT8, TRIB3) and some of which, unfortunately, only exist in mouse liver nuclei.
The result is a glut of targets with theoretical promise but limited experimental support. These tend to linger in corporate slides, sometimes festooned with 3D molecular models and glowing heatmaps, until someone asks how they actually function in vivo. Cue: awkward silence.
9. Conclusion: Smarter Arrows, Same Quiver
So, are we getting better at targeting disease? Arguably, yes. We can drug targets once thought impossible, and our understanding of biology has deepened. But for every KRAS G12C inhibitor, there are a dozen failed kinase programs. The bullseyes are real, but the arrows are still expensive and often miss.
In the end, drug targets are like the economy: complex, unpredictable, and more art than science. We pick them, drug them, and hope that biology cooperates. Occasionally, it does. When it doesn’t, we rename the failure a “platform” and go again.
The target may be a molecule, but the aim, always, is hope.
Member discussion